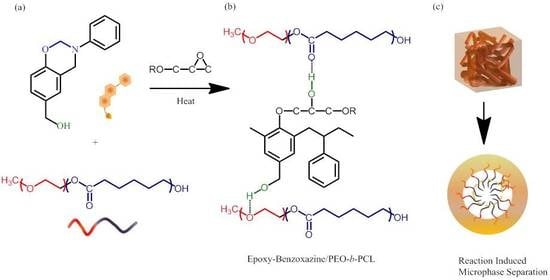
Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Flexible Epoxy Resin
2.3. Characterization
3. Results and Discussion
3.1. Analyses of PA-OH and PEO-b-PCL
3.2. Analyses of Epoxy–Benzoxazine/PEO-b-PCL Mixtures
3.3. Mechanical Properties of Epoxy-Benzoxazine/PEO-b-PCL Mixtures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocomposites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2017, 25, 1604754. [Google Scholar] [CrossRef]
- Jkramullah, S.R.; Gopakumar, D.A.; Thalib, S.; Huzni, S.; Khalil, H.P.S.A. Interfacial Compatibility Evaluation on the Fiber Treatment in the Typha Fiber Reinforced Epoxy Composites and Their Effect on the Chemical and Mechanical Properties. Polymers 2018, 10, 1316. [Google Scholar] [CrossRef]
- Allaer, K.; Baere, I.; Paegegem, W.V.; Degrieck, J. Direct fracture toughness determination of a ductile epoxy polymer from digital image correlation measurements on a single edge notched bending sample. Polym. Test. 2015, 42, 199–207. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Fan, X.; Sun, Y.; Yeo, J.C.C.; He, C. Simultaneous enhancement of strength and toughness of epoxy using POSS-Rubber core–shell nanoparticles. Compos. Sci. Technol. 2015, 118, 63–71. [Google Scholar] [CrossRef]
- Jia, L.Y.; Zhang, C.; Du, Z.J.; Li, C.J.; Li, H.Q. Preparation of Interpenetrating Polymer Networks of Epoxy/Polydimethylsiloxane in a Common Solvent of the Precursors. Polym. J. 2007, 39, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.H.; Lin, C.Y. Polysiloxane modified epoxy polymer networks—I. Graft interpenetrating polymeric networks. Eur. Polym. J. 1997, 33, 903–906. [Google Scholar] [CrossRef]
- Lan, T.; Pinnavaia, T.J. Clay-Reinforced Epoxy Nanocomposites. Chem. Mater. 1994, 6, 2216–2219. [Google Scholar] [CrossRef]
- Koh, K.L.; Ji, X.; Dasari, A.; Lu, X.; Lau, S.K.; Chen, Z. Fracture Toughness and Elastic Modulus of Epoxy-Based Nanocomposites with Dopamine-Modified Nano-Fillers. Materials 2017, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Chang, F.C. POSS related Polymer Nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wang, Y.Z.; Kuo, S.W.; Huang, C.F.; Tung, P.H.; Chang, F.C. Thermal and Dielectric Properties and Curing Kinetics of Nanomaterials Formed from POSS-Epoxy and Meta-Phenylenediamine. Polymer 2004, 45, 6897–6908. [Google Scholar] [CrossRef]
- Strachota, A.; Kroutilova, I.; Kovarova, J.; Matejka, L. Epoxy Networks Reinforced with Polyhedral Oligomeric Silsesquioxanes (POSS). Thermomechanical Properties. Macromolecules 2004, 37, 9457–9464. [Google Scholar] [CrossRef]
- Yourdkhani, M.; Liu, W.; Baril-Gosselin, S.; Robitaille, F.; Hubert, P. Carbon nanotube-reinforced carbon fibre-epoxy composites manufactured by resin film infusion. Compos. Sci. Technol. 2018, 166, 169–175. [Google Scholar] [CrossRef]
- Kadhim, N.; Mei, Y.; Wang, Y.; Li, Y.; Meng, F.; Jiang, M.; Zhou, Z. Remarkable Improvement in the Mechanical Properties of Epoxy Composites Achieved by a Small Amount of Modified Helical Carbon Nanotubes. Polymers 2018, 10, 1103. [Google Scholar] [CrossRef]
- Xu, W.; Chen, J.; Chen, S.; Chen, Q.; Lin, J.; Liu, H. Study on the Compatibilizing Effect of Janus Particles on Liquid Isoprene Rubber/Epoxy Resin Composite Materials. Ind. Eng. Chem. Res. 2017, 56, 14060–14068. [Google Scholar] [CrossRef]
- Andrews, T.; Liebig, A.; Cook, J.; Marsh, P.; Ciocanel, C.; Lindberg, G.E.; Browder, C.C. Development of a PEO-based lithium ion conductive epoxy resin polymer electrolyte. Solid State Ion. 2018, 326, 150–158. [Google Scholar] [CrossRef]
- Chen, J.L.; Chang, F.C. Phase Separation Process in Poly(ε-caprolactone)−Epoxy Blends. Macromolecules 1999, 32, 5348–5356. [Google Scholar] [CrossRef]
- Inoue, T. Reaction-induced phase decomposition in polymer blends. Prog. Polym. Sci. 1995, 20, 119–153. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured Thermosets from Self-Assembled Amphiphilic Block Copolymer/Epoxy Resin Mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Thompson, Z.J.; Hillmyer, M.A.; Liu, J.; Sue, H.J.; Dettloff, M.; Bates, F.S. Block Copolymer Toughened Epoxy: Role of Cross-Link Density. Macromolecules 2009, 42, 2333–2335. [Google Scholar] [CrossRef]
- Chu, W.-C.; Lin, W.-S.; Kuo, S.-W. Flexible Epoxy Resin Formed Upon Blending with a Triblock Copolymer through Reaction-Induced Microphase Separation. Materials 2016, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Stuparu, M.C.; Khan, A.; Hawker, C.J. Phase separation of supramolecular and dynamic block copolymers. Polym. Chem. 2012, 3, 3033–3044. [Google Scholar] [CrossRef]
- Rao, J.; Paunescu, E.; Mirmohades, M.; Gadwal, I.; Khaydarov, A.; Hawker, C.J.; Bang, J.; Khan, A. Supramolecular mimics of phase separating covalent diblock copolymers. Polym. Chem. 2012, 3, 2050–2056. [Google Scholar] [CrossRef]
- Rao, J.; Ma, H.; Baettig, J.; Woo, S.; Stuparu, M.C.; Bang, J.; Khan, A. Self-assembly of an interacting binary blend of diblock copolymers in thin films: A potential route to porous materials with reactive nanochannel chemistry. Soft Matt. 2014, 10, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xu, Z.; Zheng, S. Microphase Separation in Thermosetting Blends of Epoxy Resin and Poly(ε-caprolactone)-block-Polystyrene Block Copolymers. Macromolecules 2008, 41, 1411–1420. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, S.; Liu, T. Epoxy resin containing poly(ethylene oxide)-block-poly(ε-caprolactone) diblock copolymer: Effect of curing agents on nanostructures. Polymer 2006, 47, 7590–7600. [Google Scholar] [CrossRef]
- Huang, C.F.; Chen, W.H.; Aimi, J.; Huang, Y.S.; Venkatesan, S.; Chiang, Y.W.; Huang, S.H.; Kuo, S.W.; Chen, T. Synthesis of well-defined PCL-b-PnBA-b-PMMA ABC-type triblock copolymers: Toward the construction of nanostructures in epoxy thermosets. Polym. Chem. 2018, 9, 5644–5654. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From Microphase Separation to Self-Organized Mesoporous Phenolic Resin through Competitive Hydrogen Bonding with Double-Crystalline Diblock Copolymers of Poly(ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Li, J.G.; Chuang, C.Y.; Kuo, S.W. Transformation and Enhanced of Long-Range-Ordered Mesoporous Phenolic Resin Templated by Poly(ethylene oxide-b-ε-caprolactone) Copolymer Blending With Star Poly(ethylene oxide)-Functionalized Silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 18583–18595. [Google Scholar] [CrossRef]
- Chu, W.C.; Li, J.G.; Kuo, S.W. From Flexible to Mesoporous Polybenzoxazine Resins Templated by Poly(ethylene oxide-b-ε-caprolactone) Copolymer through Reaction Induced Microphase Separation Mechanism. RSC Adv. 2013, 3, 6485–6498. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Jeng, U.S.; Kuo, S.W. In Situ Monitoring of the Reaction-Induced Self-Assembly of Phenolic Resin Templated by Diblock Copolymers. Macrmol. Chem. Phys. 2013, 214, 2115–2123. [Google Scholar] [CrossRef]
- Chu, W.C.; Bastakoti, B.P.; Yamauchi, Y.; Kuo, S.W. Tailored Design of Bicontinuous Gyroid Mesoporous Carbon and Nitrogen-Doped Carbon from Poly (ethylene oxide-b-caprolactone) Diblock Copolymers. Chem. Eur. J. 2017, 23, 13734–13741. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines-new high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, C.Y.; Chang, F.C. Low-surface-free-energy materials based on polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers. Polymer 1999, 40, 6565–6573. [Google Scholar] [CrossRef]
- Kuo, S.W.; Liu, W.C. Synthesis and characterization of a cured epoxy resin with a benzoxazine monomer containing allyl groups. J. Appl. Polym. Sci. 2010, 117, 3121–3127. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, J.D. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Huang, J.M.; Kuo, S.W.; Lee, Y.J.; Chang, F.C. Synthesis and Characterization of a Vinyl-Terminated Benzoxazine Monomer and its Blends with Poly(ethylene oxide). J. Polym. Sci. Part B Polym. Phys. 2007, 45, 644–653. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.H. Synergism observed in polybenzoxazine and poly (ε-caprolactone) blends by dynamic mechanical and thermogravimetric analysis. Polymer 2001, 42, 6971–6979. [Google Scholar] [CrossRef]
- Su, Y.C.; Kuo, S.W.; Yei, D.R.; Xu, H.; Chang, F.C. Thermal Property and Hydrogen Bonding in Polymer Blend of Polybenzoxazine/Poly(N-vinyl-2-pyrrolidone). Polymer 2003, 44, 2187–2191. [Google Scholar] [CrossRef]
- Chu, W.C.; Li, J.G.; Wang, C.F.; Jeong, K.U.; Kuo, S.W. Self-assembled nanostructure of polybenzoxazine resins from reaction-induced microphase separation with poly(styrene-b-4-vinylpyridine) copolymer. J. Polym. Res. 2013, 20, 272. [Google Scholar] [CrossRef]
- Kwei, T. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
- Kuo, S.W.; Huang, C.F.; Chang, F.C. Study of Hydrogen Bonding Strength in Poly(ε-caprolactone) Blends by DSC and FTIR. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1348–1359. [Google Scholar] [CrossRef]
- Huang, K.W.; Tsai, L.W.; Kuo, S.W. Influence of octakis-functionalized polyhedral oligomeric silsesquioxanes on the physical properties of their polymer nanocomposites. Polymer 2009, 50, 4876–4887. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen Bonding in Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-C.; Tsai, F.-C.; Huang, C.-F.; Dai, L.; Kuo, S.-W. Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent. Polymers 2019, 11, 201. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020201
Su W-C, Tsai F-C, Huang C-F, Dai L, Kuo S-W. Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent. Polymers. 2019; 11(2):201. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020201
Chicago/Turabian StyleSu, Wei-Chen, Fang-Chang Tsai, Chih-Feng Huang, Lizong Dai, and Shiao-Wei Kuo. 2019. "Flexible Epoxy Resins Formed by Blending with the Diblock Copolymer PEO-b-PCL and Using a Hydrogen-Bonding Benzoxazine as the Curing Agent" Polymers 11, no. 2: 201. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020201