Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization
Abstract
:1. Introduction
2. Methods and Modeling Systems
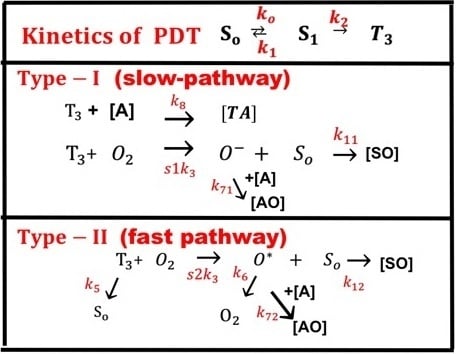
2.1. Photochemical Kinetics
2.2. Generalized Beer–Lambert Law
2.3. Efficacy Profiles
2.4. Optimal Efficacy
2.5. Depletion Time (T*)
2.6. Volume Efficacy and Crosslink Time and Depth
3. Results and Discussions
3.1. Concentration Profiles
3.2. Efficacy Profiles
3.3. Discussion of Numerical Results
3.4. The Role of Oxygen and Competing Process
3.5. Analysis of Experimental Results
4. Discussions for Optimal Strategy
4.1. Crosslink Depth and Uniformity
4.2. Optimized (C0/I0) Ratio
4.3. Transition Feature of S Function
4.4. Accelerated Photopolymerization
4.5. Strategy for Improved Efficacy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fouassier, J.-P. Photoinitiation, Photo-Polymerization, and Photocuring: Fundamentals and Applications; Hanser Gardner Publications: Munich, Germany, 1995. [Google Scholar]
- Chen, F.M.; Shi, S. Principles of Tissue Engineering, 4th ed.; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Kottisch, V.; Michaudel, Q.; Fors, B.P. Photocontrolled interconversion of cationic and radical polymerizations. J. Am. Chem. Soc. 2017, 139, 10665–10668. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, W.; Li, G. The microstructure and stability of collagen hydrogel cross-linked by glutaraldehyde. Polym. Degrad. Stab. 2016, 130, 264–270. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2018, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.Y.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tang, H.; Sun, H. Progress in photo-responsive polypeptide derived nano-assemblies. Micromachines 2018, 9, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Klok, H.A. Click chemistry in biomaterials, nanomedicine, and drug delivery. Biomacromolecules 2016, 17, 1–3. [Google Scholar] [CrossRef]
- Chen, M.C.; Garber, L.; Smoak, M.; Fragason, C.; Scherr, T.; Blackbum, C.; Bacchus, S.; Lopes, M.J.; Polman, J.A.; Hayes, D.J. In vitro and in vivo vharacterization of pentaerythritol triacrylate-co-trimethylolpropane nanocomposite scaffolds as potential bone augments and crafts. Tissue Eng. Part A 2015, 21, 320–331. [Google Scholar] [CrossRef]
- Chatani, S.; Gong, T.; Earle, B.A.; Podgoŕski, M.; Bowman, C.N. Visible-light initiated thiol-Michael addition photopolymerization reactions. ACS Macro Lett. 2014, 3, 315–318. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]
- Hansen, M.J.; Velema, W.A.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Wavelength-selective cleavage of photoprotecting. Macromolecules 2017, 50, 5652–5660. [Google Scholar]
- Zhang, X.; Xi, W.; Wang, C.; Podgorski, M.; Bowman, C.N. Visible-light-initiated thiol-Michael addition polymerizations with coumarin-based photobase generators: Another photoclick reaction strategy. ACS Macro Lett. 2016, 5, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xi, W.; Huang, S.; Long, K.; Bowman, C.N. Wavelength-selective sequential polymer network formation controlled with a two-color responsive initiation system. Macromolecules 2017, 50, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.X.; Pattanayak, S.; Wang, C.; Fairbanks, B.; Gong, T.; Wagner, J.; Kloxin, C.J.; Bowman, C.N. Clickable Nucleic Acids: Sequence-Controlled Periodic Copolymer/Oligomer Synthesis by Orthogonal Thiol-X Reactions. Angew. Chem. Int. Ed. 2015, 54, 14462–14467. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.X.; Peng, H.Y.; Aguirre-Soto, A.; Kloxin, C.J.; Stansbury, J.W.; Bowman, C.N. Spatial and Temporal Control of Thiol-Michael Addition via Photocaged Superbase in Photopatterning and Two-Stage Polymer Networks Formation. Macromolecules 2014, 47, 6159–6165. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.; Liu, H.Y.; Lin, C.C. Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine. Biomater. Sci. 2017, 5, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claudino, M.; Zhang, X.; Alim, M.D.; Podgoŕski, M.; Bowman, C.N. Mechanistic Kinetic Modeling of Thiol-Michael Addition Photopolymerizations via Photocaged “superbase” Generators: An Analytical Approach. Macromolecules 2016, 49, 8061–8074. [Google Scholar] [CrossRef]
- Konuray, A.O.; Liendo, F.; Fernández-Francos, X.; Serra, À.; Sangermano, M.; Ramis, X. Sequential curing of thiol-acetoacetate-acrylate thermosets by latent Michael addition reactions. Polymer 2017, 113, 193–199. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernandez-Francos, X.; Remis, X.; Serra, A. State of the art in dual-curing acrylate Systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef]
- Dendukuri, D.S.; Panda, P.; Haghgooie, R.; Kim, J.M.; Hatton, A.; Doyle, P.S. Modleing of oxygen-inhibited free radical photopolymerization in a PDMS microfluidic. Macromolecules 2008, 41, 8547–8556. [Google Scholar] [CrossRef]
- Terrones, G.; Pearlstein, A.J. Effects of optical attenuation and consumption of a photobleaching initiator on local initiation rates in photopolymerizations. Macromolecules 2001, 34, 3195–3204. [Google Scholar] [CrossRef]
- Kenning, N.S.; Kriks, D.; El-Maazawi, M.; Scranton, A. Spatial and temporal evolution of the photo initiation rate for thick polymer systems illuminated on both sides. Polym. Int. 2005, 54, 1429–1439. [Google Scholar]
- Miller, G.A.; Gou, L.; Narayanan, V.; Scranton, A.B. Modeling of photobleaching for the photoinitiation of thick polymerization systems. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 793–808. [Google Scholar] [CrossRef]
- Cabral, J.T.; Hudson, S.D.; Harrison, C.; Douglas, J.F. Frontal Photopolymerization for Microfluidic Applications. Langmuir 2004, 20, 10020–10029. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Prud’homme, R.K.; Aksay, J.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, M. Cure depth of photo-polymerized gels. Macromol. Theory Simul. 2014, 23, 340–352. [Google Scholar] [CrossRef]
- Huang, S.; Sinha, J.; Podgorski, M.; Zhang, X.; Claudino, M.; Bowman, C.N. Mechanistic modeling of the Thiol−Michael addition polymerization kinetics: Structural effects of the Thiol and Vinyl monomers. Macromolecules 2018, 51, 5979–5988. [Google Scholar] [CrossRef]
- Zhu, T.C.; Finlay, J.C.; Zhou, X.; Li, J. Macroscopic modeling of the singlet oxygen production during PDT. Proc. SPIE 2007, 6427, 6427O8. [Google Scholar]
- Kim, M.M.; Ghogare, A.A.; Greer, A.; Zhu, T. On the in vivo photochemical rate parameters for PDT reactive oxygen species modeling. Phys. Med. Biol. 2017, 62, R1–R48. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, H.W.; Cheng, D.C. Optimal focusing and scaling law for uniform photo-polymerization in a thick medium using a focused UV Laser. Polymers 2014, 6, 552–564. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, H.W.; Cheng, D.C. Modeling the kinetics of enhanced photo-polymerization under a collimated and a reflecting focused UV laser. Polymers 2014, 6, 1489–1501. [Google Scholar] [CrossRef]
- Lin, J.T.; Wang, K.C. Analytic formulas and numerical simulations for the dynamics of thick and non-uniform polymerization by a UV light. J. Polym. Res. 2016, 23, 53. [Google Scholar] [CrossRef]
- Lin, J.T.; Cheng, D.C. Modeling the efficacy profiles of UV-light activated corneal collagen crosslinking. PLoS ONE 2017, 12, e0175002. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T. Efficacy S-formula and kinetics of oxygen-mediated (type-II) and non-oxygen-mediated (type-I) corneal cross-linking. Ophthalmol. Res. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Lin, J.T. A Critical Review on the kinetics, efficacy, safety, nonlinear law and optimal protocols of corneal cross-linking. J. Ophthalmol. Vis. Neurosci. 2018, 3, 17–26. [Google Scholar]
- Lin, J.T. A proposed concentration-controlled new protocol for optimal corneal crosslinking efficacy in the anterior stroma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 431–432. [Google Scholar] [CrossRef] [PubMed]
- O’Brart, N.A.L.; O’Brart, D.P.S.; Aldahlawi, N.H.; Hayes, S.; Meek, K.M. An Investigation of the effects of riboflavin concentration on the efficacy of corneal cross-Linking using an enzymatic resistance model in porcine corneas. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1058–1065. [Google Scholar] [CrossRef]
- Holmes, R.; Yang, X.B.; Dunne, A.; Florea, L.; Wood, D.; Troci, G. Thiol-Ene photo-click collagen-PEG hydrogels: Impact of water-soluble photoinitiators on cell viability, gelation kinetics and rheological properties. Polymers 2017, 9, 226. [Google Scholar] [CrossRef]
- Whitely, M.E.; Robinson, J.L.; Stuebben, M.C.; Pearce, H.A.; McEnery, M.A.P.; Csogriff-Hernandes, E. Prevention of oxygen inhibition of polyHIP radical polymerization using a Thiol-based crosslinker. ACS Biomater. Sci. Eng. 2017, 13, 409–419. [Google Scholar] [CrossRef]









| PPS | photoinitiated polymerization system |
| BRL | Bunsen–Roscoe reciprocal law |
| BLL | Beer–Lambert law |
| CXL | corneal collagen crosslink |
| PDT | photodynamic therapy |
| PI | photoinitiator |
| Generalized BLL for light intensity | I(z, t) = I0 exp[−(A0 – A1t)]z |
| A0 = 2.3a’C0 + Q | |
| A1 = 2.3(a’ − b’)B’(1 − 0.5A’z)C0 | |
| B’ = aqgI0 | |
| Type-I efficacy S-functions | S = [2KC0/B]0.5E’ |
| E’ = 1 − exp(−Bt) | |
| B = aqgI0 exp(−Az) | |
| Crosslink depth (zC), first-order * | zC = (1/A)ln(Y0) |
| Y0 = KBtC0/d2 | |
| Crosslink Time (TC), first-order * | TC = T0 exp(0.5Az) |
| T0 = d/(aqgKC0I0)0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization. Polymers 2019, 11, 217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020217
Lin J-T, Liu H-W, Chen K-T, Cheng D-C. Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization. Polymers. 2019; 11(2):217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020217
Chicago/Turabian StyleLin, Jui-Teng, Hsia-Wei Liu, Kuo-Ti Chen, and Da-Chuan Cheng. 2019. "Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization" Polymers 11, no. 2: 217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11020217