A Novel Polymethyl Methacrylate Derivative Grafted with Cationic Iridium(III) Complex Units: Synthesis and Application in White Light-Emitting Diodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Equipments
2.1.1. Synthesis of 2-(pyridin-2-yl)-1H-benzo[d]imidazole (Compound 1)
2.1.2. Synthesis of 6-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)hexan-1-ol (Compound 2)
2.1.3. Synthesis of 6-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)hexyl Methacrylate (Compound 3)
2.1.4. Synthesis of the Cationic Iridium(III) Complex (Compound 4)
2.1.5. Synthesis of the Polymer (Compound 5)
2.2. Fabrication and Performance Measurements of LEDs
3. Results and Discussion
3.1. Photoluminescence Property
3.2. Thermal Stability and Thermal Quenching Property
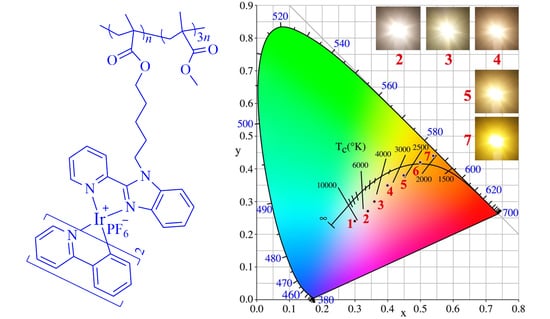
3.3. Performances of the Polymer Used in LEDs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Crawford, M.H.; Coltrin, M.E.; Fischer, A.J.; Koleske, D.D.; Subramania, G.S.; Wang, G.T.; Wierer, J.J.; Karlicek, R.F., Jr. Toward smart and ultra-efficient solid-state lighting. Adv. Opt. Mater. 2014, 2, 809–836. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, R.-S. Advances in phosphors for light-emitting diodes. J. Phys. Chem. Lett. 2011, 2, 1268–1277. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Chen, L.; Lin, C.C.; Yeh, C.W.; Liu, R.S. Light converting inorganic phosphors for white light-emitting diodes. Materials 2010, 3, 2172–2195. [Google Scholar] [CrossRef]
- Amano, H.; Kito, M.; Hiramatsu, K.; Akasaki, I. P-type conduction in Mg-doped GaN treated with low-energy electron beam irradiation (LEEBI). Jpn. J. Appl. Phys. 1989, 28, L2112–L2114. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar] [CrossRef]
- Sun, W.; Jia, Y.; Pang, R.; Li, H.; Ma, T.; Li, D.; Fu, J.; Zhang, S.; Jiang, L.; Li, C. Sr9Mg1.5(PO4)7:Eu2+: A novel broadband orange-yellow-emitting phosphor for blue light-excited warm white LEDs. ACS Appl. Mater. Interfaces 2015, 7, 25219–25226. [Google Scholar] [CrossRef]
- Wen, D.; Kuwahara, H.; Kato, H.; Kobayashi, M.; Sato, Y.; Masaki, T.; Kakihana, M. Anomalous orange light-emitting (Sr,Ba)2SiO4:Eu2+ phosphors for warm white LEDs. ACS Appl. Mater. Interfaces 2016, 8, 11615–11620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhuang, W.; Hu, Y.; Liu, R.; Jiang, Z.; Liu, Y.; Li, Y.; Zheng, Y.; Chen, L.; Zhong, J. A broad-band orange-yellow-emitting Lu2Mg2Al2Si2O12: Ce3+ phosphor for application in warm white light-emitting diodes. RSC Adv. 2017, 7, 46713–46720. [Google Scholar] [CrossRef]
- Setlur, A.A.; Lyons, R.J.; Murphy, J.E.; Kumar, N.P.; Kishore, M.S. Blue light-emitting diode phosphors based upon oxide, oxyhalide, and halide hosts. ECS J. Solid State Sci. Technol. 2013, 2, R3059–R3070. [Google Scholar] [CrossRef]
- Martino, D.D.; Beverina, L.; Sassi, M.; Brovelli, S.; Tubino, R.; Meinardi, F. Straightforward fabrication of stable white LEDs by embedding of inorganic UV-LEDs into bulk polymerizedpolymethyl-methacrylate doped with organic dyes. Sci. Rep. 2014, 4, 4400. [Google Scholar] [CrossRef]
- Findlay, N.J.; Bruckbauer, J.; Inigo, A.R.; Breig, B.; Arumugam, S.; Wallis, D.J.; Martin, R.W.; Skabara, P.J. An organic down-converting material for white-light emission from hybrid LEDs. Adv. Mater. 2014, 26, 7290–7294. [Google Scholar] [CrossRef]
- Rajamouli, B.; Sivakumar, V. White light emissive bipolar ligand and their EuIIIcomplex for white/red light emitting diodes. J. Photochem. Photobiol. A Chem. 2017, 347, 26–40. [Google Scholar]
- Meng, G.; Chen, Z.; Tang, H.; Liu, Y.; Wei, L.; Wang, Z. Application of a novel cationic iridium(III) complex as a red phosphor in warm white light-emitting diodes. New J. Chem. 2015, 39, 9535–9542. [Google Scholar] [CrossRef]
- Niklaus, L.; Dakhil, H.; Kostrzewa, M.; Coto, P.B.; Sonnewald, U.; Wierschem, A.; Costa, R.D. Easy and versatile coating approach for long-living white hybrid light-emitting diodes. Mater. Horiz. 2016, 3, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-Y.; Wang, X.-L.; Zhang, X.; Qin, C.; Li, P.; Su, Z.-M.; Zhu, D.-X.; Shan, G.-G.; Shao, K.-Z.; Wu, H.; et al. Efficient and tunable white-light emission of metal-organic frameworks by iridium-complex encapsulation. Nat. Commun. 2013, 4, 2717. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.-F.; Yu, S.-C.; Che, C.-M.; Lai, P.T. Efficient white and red light emission from GaN/tris-(8-hydroxyquinolato) aluminum/platinum(II)meso-tetrakis(pentafluorophenyl) porphyrinhybrid light-emitting diodes. Appl. Phys. Lett. 2003, 83, 1518–1520. [Google Scholar] [CrossRef]
- Zhang, C.; Heeger, A.J. Gallium nitride/conjugated polymer hybrid light emitting diodes: Performance and lifetime. J. Appl. Phys. 1998, 84, 1579–1582. [Google Scholar] [CrossRef]
- Chu, Y.; Hao, H.; Xie, H.; Chen, C.; Cai, P.; Seo, H.J. Preparation of lanthanide (Eu3+, Tb3+)-complex-grafted copolymer of methyl methacrylate and maleic anhydride films and the promising application as LED luminous layers. J. Mater. Sci. Mater. Electron. 2017, 28, 5615–5622. [Google Scholar] [CrossRef]
- Wang, M.-S.; Guo, S.-P.; Li, Y.; Cai, L.-Z.; Zou, J.-P.; Xu, G.; Zhou, W.-W.; Zheng, F.-K.; Guo, G.-C. A direct white-light-emitting metal-organic framework with tunable yellow-to-white photoluminescence by variation of excitation light. J. Am. Chem. Soc. 2009, 131, 13572–13573. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hua, Z.; Wang, G.; Uvdala, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.; Orti, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Ma, D.; Tsuboi, T.; Qiu, Y.; Duan, L. Recent progress in ionic iridium(III) complexes for organic electronic devices. Adv. Mater. 2017, 29, 1603253. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Ying, L.; Huang, F. Recent progresses of iridium complex-containing macromolecules for solution-processed organic light-emitting diodes. J. Inorg. Organomet. Polym. 2014, 24, 905–926. [Google Scholar] [CrossRef]
- Baschieri, A.; Monti, F.; Armaroli, N.; Mazzotti, G.; Giorgini, L.; Sambri, L.; Benelli, T. Luminescent methacrylic copolymers with side-chain cyclometalated iridium(III) complexes. Dyes Pigment. 2019, 160, 188–197. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Li, S.P.-Y.; Zhang, K.Y. Development of luminescent iridium(III) polypyridine complexes as chemical and biological probes. New J. Chem. 2011, 35, 265–287. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, R.; Ye, Y.; Tang, H.; Dong, X.; Yan, J.; Wang, K.; Zhou, Q.; Wang, Z. Application of a novel red-emitting cationic iridium(III) coordination polymer in warm white light-emitting diodes. Opt. Mater. 2018, 76, 141–146. [Google Scholar] [CrossRef]
- Yang, H.; Meng, G.; Zhou, Y.; Tang, H.; Zhao, J.; Wang, Z. The photoluminescent properties of new cationic iridium(III) complexes using different anions and their applications in white light-emitting diodes. Materials 2015, 8, 6105–6116. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Sun, R.; Chen, M.; Tang, H.; Dong, X.; Wang, K.; Wang, Z. Application of an orange-yellow emitting cationic iridium(III) complex in GaN-based warm white light-emitting diodes. J. Mater. Sci. Mater. Electron. 2018, 29, 1554–1561. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Chen, Q.; Chen, B.; Qiao, Q.; Yang, W.; Wu, H.; Cao, Y. Efficient yellow–green light-emitting cationic iridium complexes based on 1,10-phenanthroline derivatives containing oxadiazole-triphenylamine unit. Dyes Pigment. 2014, 100, 79–86. [Google Scholar] [CrossRef]
- Wang, H.; He, P.; Liu, S.; Shi, J.; Gong, M. A europium(III) organic ternary complex applied in fabrication of near UV-based white light-emitting diodes. Appl. Phys. B 2009, 97, 481–487. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Tang, Z.-B.; Khan, W.U.; Wang, Y. Photoluminescence study of a broad yellow-emitting phosphor K2ZrSi2O7:Bi3+. Chem. Eng. J. 2017, 313, 1082–1087. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, G.; Yang, Y.; Yang, Z.; Li, P. Energy transfer, tunable luminescence, and thermal stability of Tb3+–Sm3+-codoped Na3Bi(PO4)2 phosphors. J. Mater. Sci. 2016, 51, 6944–6954. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, W.; Qin, L.; Huang, Y.; Wei, D.; Seo, H.J. A yellow-emitting nanophosphor of Ce3+-activated aluminate Sr3LuAl2O7.5. J. Alloys Comp. 2014, 588, 540–545. [Google Scholar] [CrossRef]







| No. of LEDs | Blending Concentrations (wt %) | Luminous Efficiency (lm·W−1) | CRI | CCT (K) | λem, max (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|
| 1 | 4.0 | 5.3 | 71.2 | 10050 | (460, 583) | (0.30, 0.24) |
| 2 | 5.0 | 13.8 | 75 | 4938 | (460, 586) | (0.34, 0.27) |
| 3 | 5.5 | 18.1 | 73.6 | 3446 | (460, 586) | (0.36, 0.30) |
| 4 | 6.0 | 16.3 | 71.8 | 3093 | (460, 586) | (0.40, 0.35) |
| 5 | 7.0 | 14.8 | 63.8 | 2557 | (460, 586) | (0.45, 0.38) |
| 6 | 8.0 | 13.7 | 59.0 | 2337 | (460, 588) | (0.50, 0.42) |
| 7 | 9.0 | 12.4 | 51.6 | 2051 | (460, 590) | (0.54, 0.44) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Dong, X.; Chen, M.; Chen, Q.; Ren, M.; Wang, K.; Zhou, Q.; Wang, Z. A Novel Polymethyl Methacrylate Derivative Grafted with Cationic Iridium(III) Complex Units: Synthesis and Application in White Light-Emitting Diodes. Polymers 2019, 11, 499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030499
Tang H, Dong X, Chen M, Chen Q, Ren M, Wang K, Zhou Q, Wang Z. A Novel Polymethyl Methacrylate Derivative Grafted with Cationic Iridium(III) Complex Units: Synthesis and Application in White Light-Emitting Diodes. Polymers. 2019; 11(3):499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030499
Chicago/Turabian StyleTang, Huaijun, Xueyan Dong, Mingxian Chen, Qiuhong Chen, Mengran Ren, Kaimin Wang, Qiang Zhou, and Zhengliang Wang. 2019. "A Novel Polymethyl Methacrylate Derivative Grafted with Cationic Iridium(III) Complex Units: Synthesis and Application in White Light-Emitting Diodes" Polymers 11, no. 3: 499. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030499