Synthesis of Self-Healing Waterborne Polyurethane Systems Chain Extended with Chitosan
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Waterborne Polyurethane (WPU)
2.3. Characterization
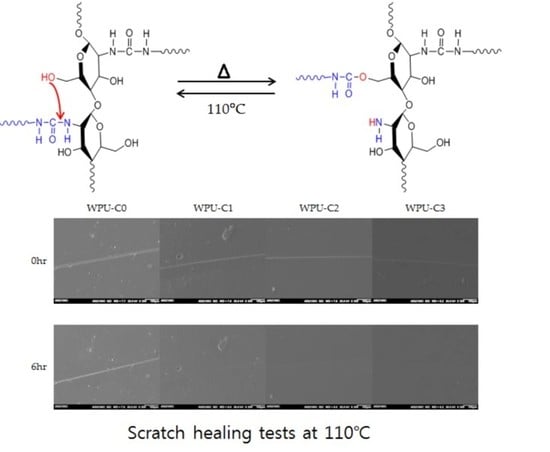
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ogliani, E.; Yu, L.; Javakhishvili, I.; Skov, A.L. A thermo-reversible silicone elastomer with remotely controlled self-healing. RSC Adv. 2018, 8, 8285–8291. [Google Scholar] [CrossRef]
- García, J.M.; Jones, G.O.; Virwani, K.; McCloskey, B.D.; Boday, D.J.; ter Huurne, G.M.; Horn, H.W.; Coady, D.J.; Bintaleb, A.M.; Alabdulrahman, A.M. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science 2014, 344, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xie, Z.; Xu, J.; Weng, Y.; Guo, B.-H. Design of a self-healing cross-linked polyurea with dynamic cross-links based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2018, 107, 249–257. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, A.S.; Baek, K.Y.; Lee, H.; Hwang, S.S. Multi-crosslinkable self-healing polysilsesquioxanes for the smart recovery of anti-scratch properties. Polymer 2017, 124, 78–87. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic Schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Polgar, L.M.; Criscitiello, F.; van Essen, M.; Araya-Hermosilla, R.; Migliore, N.; Lenti, M.; Raffa, P.; Picchioni, F.; Pucci, A. Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes. Nanomaterials (Basel) 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, A.W.; Boinard, P.; Liggat, J.J. The influence of diol chain extender on morphology and properties of thermally-triggered UV-stable self-healing polyurethane coatings. Prog. Org. Coat. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Jian, X.; Hu, Y.; Zhou, W.; Xiao, L. Self-healing polyurethane based on disulfide bond and hydrogen bond. Polym. Adv. Technol. 2018, 29, 463–469. [Google Scholar] [CrossRef]
- Collins, J.; Nadgorny, M.; Xiao, Z.; Connal, L.A. Doubly Dynamic Self-Healing Materials Based on Oxime Click Chemistry and Boronic Acids. Macromol. Rapid Commun. 2017, 38, 1600760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lv, L.; Deng, Y.; Wang, C. Self-Healing Gelatin Hydrogels Cross-Linked by Combining Multiple Hydrogen Bonding and Ionic Coordination. Macromol. Rapid Commun. 2017, 38, 1700018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Rong, M.Z. Intrinsic self-healing of covalent polymers through bond reconnection towards strength restoration. Polym. Chem. 2013, 4, 4878–4884. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Wang, Z.; Xia, H. Diels–Alder dynamic crosslinked polyurethane/polydopamine composites with NIR triggered self-healing function. Polym. Chem. 2018, 9, 2166–2172. [Google Scholar] [CrossRef]
- Gao, W.T.; Bie, M.Y.; Quan, Y.W.; Zhu, J.Y.; Zhang, W.Q. Self-healing, reprocessing and sealing abilities of polysulfide-based polyurethane. Polymer 2018, 151, 27–33. [Google Scholar] [CrossRef]
- Zhang, L.H.; Chen, L.F.; Rowan, S.J. Trapping Dynamic Disulfide Bonds in the Hard Segments of Thermoplastic Polyurethane Elastomers. Macromol. Chem. Phys. 2017, 218, 1600320. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ying, H.; Hart, K.R.; Wu, Y.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and Recyclable Poly(urea-urethane) Thermosets bearing Hindered Urea Bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, C.; Yu, C.; Fei, G.; Wang, Z.; Xia, H. A Facile Strategy for Self-Healing Polyurethanes Containing Multiple Metal-Ligand Bonds. Macromol. Rapid Commun. 2018, 39, e1700678. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, S.I.S.; Kong, J.H.; Lu, X.H. Aqueous-Only, Green Route to Self-Healable, UV-Resistant, and Electrically Conductive Polyurethane/Graphene/Lignin Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Lin, C.H.; Sheng, D.K.; Liu, X.D.; Xu, S.B.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y.M. A self-healable nanocomposite based on dual-crosslinked Graphene Oxide/Polyurethane. Polymer 2017, 127, 241–250. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D.J. Mechanical enhancement of self-healing waterborne polyurethane by graphene oxide. Prog. Org. Coat. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Erice, A.; de Luzuriaga, A.R.; Matxain, J.M.; Ruiperez, F.; Asua, J.M.; Grande, H.J.; Rekondo, A. Reprocessable and recyclable crosslinked poly(urea-urethane)s based on dynamic amine/urea exchange. Polymer 2018, 145, 127–136. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Sorbitol as a Chain Extender of Polyurethane Prepolymers to Prepare Self-Healable and Robust Polyhydroxyurethane Elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Chen, D.J. Synthesis and properties of self-healing waterborne polyurethanes containing disulfide bonds in the main chain. J. Mater. Sci. 2017, 52, 197–207. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Martin, L.; Aramburu, N.; Irusta, L.; Fernandez-Berridi, M.J. Coumarin based light responsive healable waterborne polyurethanes. Prog. Org. Coat. 2016, 99, 314–321. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, H.D. Synthesis and characterization of waterborne polyurethane adhesives containing different amount of ionic groups (I). J. Appl. Polym. Sci. 2006, 102, 5684–5691. [Google Scholar] [CrossRef]
- Fu, H.Q.; Wang, Y.; Chen, W.F.; Xiao, J. Reinforcement of waterborne polyurethane with chitosan-modified halloysite nanotubes. Appl. Surf. Sci. 2015, 346, 372–378. [Google Scholar] [CrossRef]
- Xu, D.; Meng, Z.; Han, M.; Xi, K.; Jia, X.; Yu, X.; Chen, Q. Novel blood-compatible waterborne polyurethane using chitosan as an extender. J. Appl. Polym. Sci. 2008, 109, 240–246. [Google Scholar] [CrossRef]
- Ghosh, B.; Chellappan, K.V.; Urban, M.W. Self-healing inside a scratch of oxetane-substituted chitosan-polyurethane (OXE-CHI-PUR) networks. J. Mater. Chem. 2011, 21, 14473–14486. [Google Scholar] [CrossRef]
- Kittur, F.; Prashanth, K.H.; Sankar, K.U.; Tharanathan, R. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Sarva, S.S.; Hsieh, A.J. The effect of microstructure on the rate-dependent stress–strain behavior of poly (urethane urea) elastomers. Polymer 2009, 50, 3007–3015. [Google Scholar] [CrossRef]
- Lei, L.; Zhong, L.; Lin, X.Q.; Li, Y.Y.; Xia, Z.B. Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink. Chem. Eng. J. 2014, 253, 518–525. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. The phase mixing studies on moisture cured polyurethane-ureas during cure. Polymer 2006, 47, 3814–3825. [Google Scholar] [CrossRef]








| Sample Code | Composition (by wt. %) | Chain Extender Ratio (in Mols) | |||||
|---|---|---|---|---|---|---|---|
| DMPA | PTMEG | IPDI | EDA | Chitosan | EDA | Chitosan | |
| WPU-C0 | 6.00 | 57.02 | 32.57 | 4.40 | 0.00 | 1.0 | 0.0 |
| WPU-C1 | 6.00 | 55.05 | 32.13 | 3.90 | 2.89 | 0.9 | 0.1 |
| WPU-C2 | 6.00 | 53.15 | 31.71 | 3.43 | 5.70 | 0.8 | 0.2 |
| WPU-C3 | 6.00 | 51.29 | 31.30 | 2.96 | 8.44 | 0.7 | 0.3 |
| Sample Code | Urethane C=O (1697 cm−1)/Urea C=O (1644 cm−1) | % Increase after Heating |
|---|---|---|
| WPU-C0 | 0.97 | |
| WPU-C0-treated | 1.00 | 3 |
| WPU-C1 | 1.05 | |
| WPU-C1-treated | 1.13 | 8 |
| WPU-C2 | 0.93 | |
| WPU-C2-treated | 1.11 | 19 |
| WPU-C3 | 0.89 | |
| WPU-C3-treated | 1.04 | 17 |
| Sample Code | Stress (MPa) | Efficiency (%) | |
|---|---|---|---|
| Before Healing | After Healing | ||
| WPU-C0 | 17.56 | 0.74 | 4 |
| WPU-C1 | 30.75 | 10.73 | 35 |
| WPU-C2 | 23.26 | 10.37 | 45 |
| WPU-C3 | 13.37 | 6.25 | 47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-I.; Kim, S.-H.; Lee, D.-S. Synthesis of Self-Healing Waterborne Polyurethane Systems Chain Extended with Chitosan. Polymers 2019, 11, 503. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030503
Lee D-I, Kim S-H, Lee D-S. Synthesis of Self-Healing Waterborne Polyurethane Systems Chain Extended with Chitosan. Polymers. 2019; 11(3):503. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030503
Chicago/Turabian StyleLee, Dae-Il, Seung-Hyun Kim, and Dai-Soo Lee. 2019. "Synthesis of Self-Healing Waterborne Polyurethane Systems Chain Extended with Chitosan" Polymers 11, no. 3: 503. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11030503