Photophysical and Electroluminescence Characteristics of Polyfluorene Derivatives with Triphenylamine
Abstract
:1. Introduction
2. Experimental
2.1. Polymer Synthesis
2.2. OLED Fabrication
3. Results and Discussion
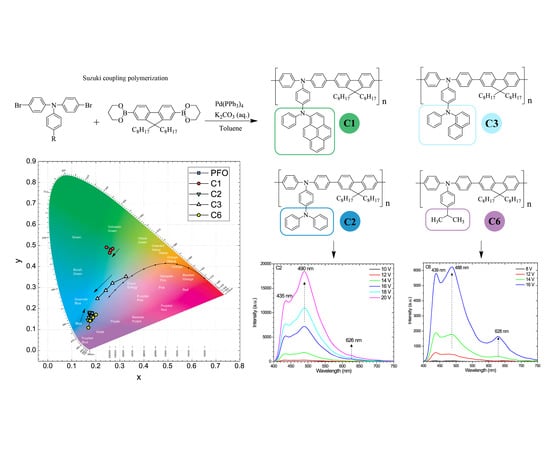
3.1. Absorption and Photoluminescence Spectra
3.2. Electroluminescence Characteristics
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Zhao, C.E.; Lin, Z.Q.; Gu, P.Y.; Zhang, Q. Nanostructured conjugated polymers for energy-related applications beyond solar cells. Chem. Asian J. 2016, 11, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-polymer-amplified sensing, imaging, and therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef]
- Yao, C.-J.; Zhang, H.-L.; Zhang, Q. Recent progress in thermoelectric materials based on conjugated polymers. Polymers 2019, 11, 107. [Google Scholar] [CrossRef]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured conjugated polymers: Toward high-performance organic electrodes for rechargeable batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Morin, P.O.; Bura, T.; Leclerc, M. Realizing the full potential of conjugated polymers: Innovation in polymer synthesis. Mater. Horizons. 2016, 3, 11–20. [Google Scholar] [CrossRef]
- Kertesz, M.; Choi, C.H.; Yang, S. Conjugated polymers and aromaticity. Chem. Rev. 2005, 105, 3448–3481. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Bredas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Berggren, M.; Inganas, O.; Gustafsson, G.; Rasmusson, J.; Andersson, M.R.; Hjertberg, T.; Wennerström, O. Light-emitting-diodes with variable colors from polymer blends. Nature 1994, 372, 444–446. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Leclerc, M.; Beaupré, S. Solar-energy production and energy-efficient lighting: Photovoltaic devices and white-light-emitting diodes using poly(2,7-fluorene), poly(2,7-carbazole), and poly(2,7-dibenzosilole) derivatives. Adv. Mater. 2010, 22, E6–E27. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Hu, S.; He, R.; Yang, W.; Wu, H.; Peng, J.; Xia, R.; Bradley, D.D.C. Red, green, and blue light-emitting polyfluorenes containing a dibenzothiophene-S,S-dioxide unit and efficient high-color-rendering-index white-light-emitting diodes made therefrom. Adv. Funct. Mater. 2013, 23, 4366–4376. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Cheng, Y.; Geng, Y.; Wang, L.; Ma, D.; Jing, X.B.; Wang, F.S. The first single polymer with simultaneous blue, green, and red emission for white electroluminescence. Adv. Mater. 2005, 17, 2974–2978. [Google Scholar] [CrossRef]
- Liu, C.-F.; Jiu, Y.; Wang, J.; Yi, J.; Zhang, X.-W.; Lai, W.-Y.; Huang, W. Star-shaped single-polymer systems with simultaneous rgb emission: Design, synthesis, saturated white electroluminescence, and amplified spontaneous emission. Macromolecules 2016, 49, 2549–2558. [Google Scholar] [CrossRef]
- Wu, F.I.; Yang, X.H.; Neher, D.; Dodda, R.; Tseng, Y.H.; Shu, C.F. Efficient white-electrophosphorescent devices based on a single polyfluorene copolymer. Adv. Funct. Mater. 2007, 17, 1085–1092. [Google Scholar] [CrossRef]
- Lee, P.I.; Hsu, S.L.C.; Lin, P. White-light-emitting diodes from single polymer systems based on polyfluorene copolymers with quinoxaline derivatives. Macromolecules 2010, 43, 8051–8057. [Google Scholar] [CrossRef]
- Chen, W.; Wang, K.; Hung, W.; Jiang, J.; Liaw, D.-J.; Lee, K.-R.; Lai, J.Y.; Chen, C.L. Novel triarylamine-based alternating conjugated polymer with high hole mobility: Synthesis, electro-optical, and electronic properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4654–4667. [Google Scholar] [CrossRef]
- Tang, R.; Tan, Z.; Li, Y.; Xi, F. Synthesis of new conjugated polyfluorene derivatives bearing triphenylamine moiety through a vinylene bridge and their stable blue electroluminescence. Chem. Mater. 2006, 18, 1053–1061. [Google Scholar] [CrossRef]
- Giovanella, U.; Pasini, M.; Destri, S.; Porzio, W.; Botta, C. Stabilized blue emission from polyfluorene-based light-emitting diodes: The role of triphenylamine. Synth. Met. 2008, 158, 113–119. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, J.H.; Hwang, D.H. Synthesis and light-emitting properties of new polyfluorene copolymers containing a triphenylamine based hydrazone comonomer. Curr. Appl. Phys. 2006, 6, 752–755. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, C.Y.; Abidin, T.; Jiang, J.C.; Shie, W.R.; Li, L.J.; Liaw, D.J. Transmissive-to-black fast electrochromic switching from a long conjugated pendant group and a highly dispersed polymer/SWNT. Polym. Chem. 2018, 9, 619–626. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, K.L.; Liaw, D.J.; Lee, K.R.; Lai, J.Y. Electrochromic material containing unsymmetrical substituted N,N,N0,N0-Tetraaryl-1,4-phenylenediamine: Synthesis and their optical, electrochemical, and electrochromic properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1469–1476. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.H.; Lu, P.; Yang, Y. Fluorescent, carrier-trapping dopants for highly efficient single-layer polyfluorene LEDs. Adv. Funct. Mater. 2007, 17, 2203–2210. [Google Scholar] [CrossRef]
- Kalinowski, J.; Giro, G.; Cocchi, M.; Fattori, V.; Di Marco, P. Unusual disparity in electroluminescence and photoluminescence spectra of vacuum-evaporated films of 1,1-bis ((di-4-tolylamino) phenyl) cyclohexane. Appl. Phys. Lett. 2000, 76, 2352–2354. [Google Scholar] [CrossRef]
- Palilis, L.C.; Lidzey, D.G.; Redecker, M.; Inbasekaran, M.; Woo, E.P.; Wu, W.W. Bright and efficient blue and green light-emitting diodes based on conjugated polymer blends. Synth. Met. 2000, 111, 159–163. [Google Scholar] [CrossRef]
- Bliznyuk, V.N.; Carter, S.A.; Scott, J.C.; Klärner, G.; Miller, R.D.; Miller, D.C. Electrical and photoinduced degradation of polyfluorene based films and light-emitting devices. Macromolecules 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Yu, W.-L.; Pei, J.; Huang, W.; Heeger, A.J. A novel triarylamine-based conjugated polymer and its unusual light-emitting properties. Chem. Commun. 2000, 8, 681–682. [Google Scholar] [CrossRef]
- List, E.J.W.; Gaal, M.; Guentner, R.; De Freitas, P.S.; Scherf, U. The role of keto defect sites for the emission properties of polyfluorene-type materials. Synth. Met. 2003, 139, 759–763. [Google Scholar] [CrossRef]
- Rybakiewicz, R.; Zagorska, M.; Pron, A. Triphenylamine-based electroactive compounds: Synthesis, properties and application to organic electronics. Chem. Pap. 2017, 71, 243–268. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, K.L.; Liaw, D.J.; Lee, K.R.; Lai, J.Y. N,N,N′,N′-tetraphenyl-1,4-phenylenediamine-fluorene alternating conjugated polymer: Synthesis, characterization, and electrochromic application. Macromolecules 2010, 43, 2236–2243. [Google Scholar] [CrossRef]
- Rothe, C.; Monkman, A. Triplet exciton migration in a conjugated polyfluorene. Phys. Rev. B 2003, 68, 1–11. [Google Scholar] [CrossRef]
- Wilson, J.S.; Dhoot, A.S.; Seeley, A.J.; Khan, M.S.; Köhler, A.; Friend, R.H. Spin-dependent exciton formation in pi-conjugated compounds. Nature 2001, 413, 828–831. [Google Scholar] [CrossRef]









| Polymer | HOMO a (eV) | LUMO a (eV) | Eg b (eV) |
|---|---|---|---|
| C1c | −4.96 | −2.20 | 2.76 |
| C2c | −5.03 | −2.19 | 2.84 |
| C3c | −4.86 | −2.08 | 2.78 |
| C6 | −5.16 | −2.26 | 2.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, P.-I.; Ong, G.L.; Tan, S.H.; Tan, Z.W.; Hii, Y.H.; Wong, Y.L.; Cheah, K.S.; Yap, S.L.; Ong, T.S.; et al. Photophysical and Electroluminescence Characteristics of Polyfluorene Derivatives with Triphenylamine. Polymers 2019, 11, 840. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050840
Zhang Q, Wang P-I, Ong GL, Tan SH, Tan ZW, Hii YH, Wong YL, Cheah KS, Yap SL, Ong TS, et al. Photophysical and Electroluminescence Characteristics of Polyfluorene Derivatives with Triphenylamine. Polymers. 2019; 11(5):840. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050840
Chicago/Turabian StyleZhang, Qiang, Po-I. Wang, Guang Liang Ong, Shen Hoong Tan, Zhong Wei Tan, Yew Han Hii, Yee Lin Wong, Khee Sang Cheah, Seong Ling Yap, Teng Sian Ong, and et al. 2019. "Photophysical and Electroluminescence Characteristics of Polyfluorene Derivatives with Triphenylamine" Polymers 11, no. 5: 840. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050840