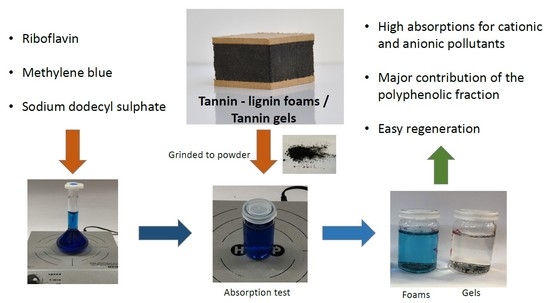
Pollutant Absorption as a Possible End-Of-Life Solution for Polyphenolic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Adsorbent Preparation
2.3. Adsorption Experiments
2.4. ATR FT-IR Investigation
2.5. Solid State 13C-NMR
2.6. Regeneration of the Adsorbents
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pollutant Removal
3.2. Adsorbent Characterization
3.3. Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pala, A.; Tokat, E. Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Res. 2002, 36, 2920–2925. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K.; Alam, S. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract. Chem. Eng. J. 2011, 174, 556–563. [Google Scholar] [CrossRef]
- Kawakita, H.; Abe, M.; Inoue, J.I.; Ohto, K.; Harada, H.; Inoue, K. Selective gold recovery using orange waste. Sep. Sci. Technol. 2009, 44, 2797–2805. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Kajiyama, K.; Ohto, K.; Harada, H.; Inoue, K. Recovery of gold from hydrochloric acid by using lemon peel gel. Sep. Sci. Technol. 2008, 43, 2363–2374. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Kajiyama, K. Persimmon peel gel for the selective recovery of gold. Hydrometallurgy 2007, 87, 133–139. [Google Scholar] [CrossRef]
- Alila, S.; Boufi, S. Removal of organic pollutants from water by modified cellulose fibres. Ind. Crops Prod. 2009, 30, 93–104. [Google Scholar] [CrossRef]
- Janoš, P.; Coskun, S.; Pilařová, V.; Rejnek, J. Removal of basic (Methylene Blue) and acid (Egacid Orange) dyes from waters by sorption on chemically treated wood shavings. Bioresour. Technol. 2009, 100, 1450–1453. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Xu, G.; Pei, Y. Effective removing of methylene blue from aqueous solution by tannins immobilized on cellulose microfibers. Int. J. Biol. Macromol. 2019, 129, 198–206. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Zhu, Y.; Wang, S.; Wang, X.; Lv, K. Novel Polymer Material for Efficiently Removing Methylene Blue, Cu(II) and Emulsified Oil Droplets from Water Simultaneously. Polymers (Basel) 2018, 10, 1393. [Google Scholar] [CrossRef]
- Yu, K.; Wang, D.; Wang, Q.; Yu, K.; Wang, D.; Wang, Q. Tough and Self-Healable Nanocomposite Hydrogels for Repeatable Water Treatment. Polymers (Basel) 2018, 10, 880. [Google Scholar] [CrossRef]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Hu, G.; Jamal, L.; Alam S. M., J. Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions. Polymers (Basel) 2019, 11, 607. [Google Scholar] [CrossRef]
- Delforno, T.P.; Moura, A.G.L.; Okada, D.Y.; Sakamoto, I.K.; Varesche, M.B.A. Microbial diversity and the implications of sulfide levels in an anaerobic reactor used to remove an anionic surfactant from laundry wastewater. Bioresour. Technol. 2015, 192, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Karray, F.; Mezghani, M.; Mhiri, N.; Djelassi, B.; Sayadi, S. Scale-down studies of membrane bioreactor degrading anionic surfactants wastewater: Isolation of new anionic-surfactant degrading bacteria. Int. Biodeterior. Biodegradation 2016, 114, 14–23. [Google Scholar] [CrossRef]
- Hussain, S.; Malik, A.H.; Iyer, P.K. Highly Precise Detection, Discrimination, and Removal of Anionic Surfactants over the Full pH Range via Cationic Conjugated Polymer: An Efficient Strategy to Facilitate Illicit-Drug Analysis. ACS Appl. Mater. Interfaces 2015, 7, 3189–3198. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manage. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater – Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chemie Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Pizzi, A.; Tondi, G.; Pasch, H.; Celzard, A. Matrix-assisted laser desorption/ionization time-of-flight structure determination of complex thermoset networks: Polyflavonoid tannin-furanic rigid foams. J. Appl. Polym. Sci. 2008, 110, 1451–1456. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Carmona-Murillo, C. Adsorbents from Schinopsis balansae: Optimisation of significant variables. Ind. Crops Prod. 2011, 33, 409–417. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Delgado-Regaña, A.; Rodríguez-González, M.A.; Rubio-Alonso, F. Optimization of tannin rigid foam as adsorbents for wastewater treatment. Ind. Crops Prod. 2013, 49, 507–514. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; González-Velasco, M.; Beltrán-Heredia, J. Acacia mearnsii de Wild Tannin-Based Flocculant in Surface Water Treatment. J. Wood Chem. Technol. 2009, 29, 119–135. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; González-Velasco, M.; Beltrán-Heredia, J.; Gragera-Carvajal, J.; Salguero-Fernández, J. Novel tannin-based adsorbent in removing cationic dye (Methylene Blue) from aqueous solution. Kinetics and equilibrium studies. J. Hazard. Mater. 2010, 174, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Paudyal, H.; Nakagawa, H.; Kawakita, H.; Ohto, K. Selective adsorption of chromium(VI) from zinc(II) and other metal ions using persimmon waste gel. Hydrometallurgy 2010, 104, 123–128. [Google Scholar] [CrossRef]
- Tondi, G.; Oo, C.W.; Pizzi, A.; Trosa, A.; Thevenon, M.F. Metal adsorption of tannin based rigid foams. Ind. Crops Prod. 2009, 29, 336–340. [Google Scholar] [CrossRef]
- Link, M.; Kolbitsch, C.; Tondi, G.; Ebner, M.; Wieland, S.; Petutschnigg, A. Formaldehyde-free tannin-based foams and their use as lightweight panels. BioResources 2011, 6, 4218–4228. [Google Scholar]
- Tondi, G.; Link, M.; Kolbitsch, C.; Lesacher, R.; Petutschnigg, A. Pilot plant up-scaling of tannin foams. Ind. Crops Prod. 2016, 79, 211–218. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin–lignin aerogels. Ind. Crops Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-based rigid foams: Characterization and modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- Kolbitsch, C.; Link, M.; Petutschnigg, A.; Wieland, S.; Tondi, G. Microwave Produced Tannin-furanic Foams. J. Mater. Sci. Res. 2012, 1. [Google Scholar] [CrossRef]
- Sepperer, T.; Petutschnigg, A.; Bogner, B.; Oostingh, G.; Hernandez-Ramos, F.; Labidi, J.; Tondi, G. Purification of industrial tannin extract through simple solid-liquid extractions - submitted. Ind. Crops Prod. 2018. [Google Scholar]
- Missio, A.L.; Tischer, B.; dos Santos, P.S.B.; Codevilla, C.; de Menezes, C.R.; Barin, J.S.; Haselein, C.R.; Labidi, J.; Gatto, D.A.; Petutschnigg, A.; et al. Analytical characterization of purified mimosa (Acacia mearnsii) industrial tannin extract: Single and sequential fractionation. Sep. Purif. Technol. 2017, 186, 218–225. [Google Scholar] [CrossRef]
- Sepperer, T.; Tondi, G. Tannin-furanic foams from purified extracts. In Proceedings of the 5th International Conference on Processing Technologies for the Forest and Biobased Industries, PTF-BPI, Freising/Munich, Germany, 20–21 September 2018. [Google Scholar]
- Tondi, G.; Link, M.; Kolbitsch, C.; Gavino, J.; Luckeneder, P.; Petutschnigg, A.; Herchl, R.; Van Doorslaer, C. Lignin-based Foams: Production Process and Characterization. BioResources 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Shan, W.; Ren, F.; Zhang, Q.; Wan, L.; Xing, Z.; Lou, Z.; Xiong, Y. Enhanced adsorption capacity and selectivity towards molybdenum in wastewater by a persimmon tannin waste based new adsorbent. J. Chem. Technol. Biotechnol. 2015, 90, 888–895. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wu, Y.; Tan, H.; Meng, F.; Wang, Y.S.; Li, M.; Zhao, L.; Liu, L.; Qian, Y.; et al. Analysis of accumulation patterns and preliminary study on the condensation mechanism of proanthocyanidins in the tea plant [Camellia sinensis]. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Bhuyan, A.K. On the mechanism of SDS-induced protein denaturation. Biopolymers 2010, 93, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, S.; Maibach, H.I. Sodium Lauryl Sulfate-Induced Irritation in the Human Face: Regional and Age-Related Differences. Skin Pharmacol. Physiol. 2006, 19, 177–180. [Google Scholar] [CrossRef]
- Hayashi, K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal. Biochem. 1975, 67, 503–506. [Google Scholar] [CrossRef]
- Banfi, D.; Patiny, L. Resurrecting and Processing NMR Spectra On-line. Chim. Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDBConstructing a Free Chemical Information System with Open-Source Components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and accurate algorithm for the simulation of NMR spectra of large spin systems. J. Magn. Reson. 2011, 209, 123–130. [Google Scholar] [CrossRef]
- Doğan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, G. Preparation and characterization of high surface area activated carbon fibers from lignin. Polymers (Basel) 2016, 8. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R. Interaction between flavonoid, quercetin and surfactant aggregates with different charges. J. Colloid Interface Sci. 2006, 302, 625–632. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Oo, C.W.; Petutschnigg, A. A simple approach to distinguish classic and formaldehyde-free tannin based rigid foams by ATR FT-IR. J. Spectrosc. 2015, 2015. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Taylor, P.; Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. 2015, 50, 37–41. [Google Scholar]
- Tondi, G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers (Basel) 2017, 9, 223. [Google Scholar] [CrossRef]
- Tondi, G.; Sepperer, T.; Cefarin, N.; Musso, M.; Reyer, A.; Vaccari, L. Multi-technique investigation of poly-furfuryl alcohol - submitted. Macromolucules 2019. [Google Scholar]
- Pizzi, A. Tannin based Wood Adhesives Technology. In Advanced Wood Adhesives Technology; Decker: New York, NY, USA, 1994; p. 304. ISBN 9780824792664. [Google Scholar]
- Pizzi, A.; Stephanou, A. A comparative C13 NMR study of polyflavonoid tannin extracts for phenolic polycondensates. J. Appl. Polym. Sci. 1993, 50, 2105–2113. [Google Scholar] [CrossRef]




| Foam | Tannin [%] | FOH [%] | DEE [%] | H2O [%] | H2SO4 [%] | CH2O/BL [%] | Temperature [°C] |
|---|---|---|---|---|---|---|---|
| Standard | 41.9 | 25.9 | 5.6 | 8.4 | 18.2 | - | 90 |
| Acetone soluble | 41.9 | 25.9 | 5.6 | 8.4 | 18.2 | - | 90 |
| Acetone insoluble | 41.9 | 25.9 | 5.6 | 8.4 | 18.2 | - | 90 |
| Tannin-Lignin | 29.6 | 20.7 | 3.3 | - | 16.8 | 29.6 | 120 |
| Formaldehyde | 44.3 | 27.5 | 5.9 | - | 13.4 | 8.9 | 45 |
| Foam | Riboflavin 1 | SDS 1 | Methylene blue 1 |
|---|---|---|---|
| Standard | 3.8 ± 0.81 a,b | 24.1 ± 0.31 a | 45.4 ± 0.31 a |
| Acetone soluble | 4.8 ± 0.88 a,b,c | 20.3 ± 0.34 a | 33.2 ± 1.29 b |
| Acetone insoluble | 7.6 ± 0.28 c | 32.7 ± 2.05 b | 63.3 ± 2.33 c |
| Tannin-Lignin | 6.0 ± 1.41 b,c | 32.8 ± 2.46 c | 73.8 ± 2.40 d |
| Formaldehyde | 2.1 ± 1.48 a | 32.6 ± 2.05 b | 56.9 ± 1.92 e |
| Foam Condition | Removal Capacity [mg/g] | Compared to Initial Removal [%] |
|---|---|---|
| Fresh | 45.38 ± 0.31 | - |
| Washed at 21 °C | 44.03 ± 0.21 | 97.6 |
| Washed at 50 °C | 44.96 ± 0.58 | 99.2 |
| Washed at 79 °C | 44.77 ± 0.48 | 98.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepperer, T.; Neubauer, J.; Eckardt, J.; Schnabel, T.; Petutschnigg, A.; Tondi, G. Pollutant Absorption as a Possible End-Of-Life Solution for Polyphenolic Polymers. Polymers 2019, 11, 911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050911
Sepperer T, Neubauer J, Eckardt J, Schnabel T, Petutschnigg A, Tondi G. Pollutant Absorption as a Possible End-Of-Life Solution for Polyphenolic Polymers. Polymers. 2019; 11(5):911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050911
Chicago/Turabian StyleSepperer, Thomas, Jonas Neubauer, Jonas Eckardt, Thomas Schnabel, Alexander Petutschnigg, and Gianluca Tondi. 2019. "Pollutant Absorption as a Possible End-Of-Life Solution for Polyphenolic Polymers" Polymers 11, no. 5: 911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050911