3.1. Kinetics and Thermodynamics of Diels–Alder Reversible Reaction
We studied kinetics of the DA reaction of tetrafunctional furan monomer F4D2000 with three maleimide monomers. The aromatic DPBMI, aliphatic HBMI, and the bismaleimide PPO3BMI with poly(oxypropylene) short chain were used and the effect of a maleimide structure was determined. It is known [
30,
31] that addition of the appropriate substituents in furan and maleimide compounds can significantly affect reactivity and reversibility of the cycloaddition reaction. The kinetics of the reversible reaction at different temperatures was followed by FTIR both in the solution (6% in dioxane) and in the bulk system. The maleimide conversion was monitored only because the conversions of maleimide and furan are equal during the reaction at the stoichiometric composition and a limited temperature. The temperature dependence of the forward DA and the reverse retro-DA (rDA) reaction rates, as well as the equilibrium position were evaluated. The data are given in the
Supplementary Materials Table S1. The reaction was followed in the temperature range of 20 to 120 °C in order to avoid the homopolymerization of maleimides occurring at a higher temperature [
15,
32].
The reaction rate of cycloaddition to form the DA cyclohexene adduct increases with increasing temperature, however the equilibrium conversion decreases due to the rDA reaction dominating at a high temperature (see
Figure 1). In the solution, the equilibrium conversion at room temperature reaches the high value,
αe = 0.82–0.90, while at 120 °C the reversible reaction is shifted to the monomers and
αe = 0.47–0.50. The determined activation energies,
Ea,DA = 32–38 kJ/mol,
Ea,rDA = 65–81 kJ/mol, are slightly lower compared to literature data [
3,
12,
15,
33], however they are in accordance with the data obtained using polymeric systems [
19,
20]. Moreover, the DA reaction is known to involve the effect of stereochemistry. Both exo- and endo-isomer adducts are formed differing in the rate of formation and thermodynamic stability [
33,
34]. This effect, however, was not taken into account in the evaluation of the kinetics because the FTIR measurement does not allow to distinguish between these isomers.
The reaction kinetics in bulk was followed in a solvent-free mixture of monomers in case of the homogeneous system F4D2000–PPO3BMI. The maleimides HBMI and DPBMI are crystalline and the initial monomer mixtures are heterogeneous. In order to eliminate the effect of phase separation, the procedure starting from a cross-linked network was used.
In the case of HBMI the cured network is homogeneous due to the reaction blending and the mixture with DPBMI is homogenized by melting of the bismaleimide at 120 °C. The cured networks were heated for 30 min at 120 °C to promote the rDA reaction and to break down the DA covalent bonds. The partially split up structure was cooled down to a particular temperature and the kinetics of the isothermal bonds reformation was followed by FTIR as illustrated in
Figure 2. The reaction starts from a relatively high conversion, due to the incompletely broken structure at 120 °C. The reaction in bulk was found to show higher rate constants than in a dioxane solution (See
Supplementary Material Table S1). Taking into account the rate constants and the concentration of functional groups in bulk systems, the reaction rates of maleimides in the network decrease in the series for the forward DA reaction are HBMI > DPBMI > PPO3BMI, and for the reverse rDA reaction are DPBMI > PPO3BMI > HBMI, respectively.
The equilibrium conversion in the solid state in the networks and its temperature dependence was determined by annealing the corresponding network under isothermal conditions at different temperatures. The results reveal that the equilibrium position is dependent on the maleimide structure. Moreover, the conversions are higher in solid networks compared to the reaction in solution (see
Figure 3), as found also by other groups [
3,
15,
33]. The DA cycloadduct is relatively stable at temperatures below 70 °C. The retro-DA reaction is insignificant at
T < 70 °C, mainly in solid networks. Particularly the HBMI involving network exhibits the high equilibrium conversion. In this case, the cyclohexene adduct is stable up to very high temperatures. The equilibrium conversion
αe > 0.90 at
T = 70 °C and it is 0.60 even at 120 °C.
The results are in a general agreement with the work of Boutelle et al. [
30] describing the effect of maleimide substituents on the furan–maleimide DA reaction. Both the N-alkyl and N-phenyl substituents increase exergonicity of the reaction compared to the unsubstituted maleimide. The alkyl substitution shows the greatest effect as the more negative reaction free energy was reported compared to the N-phenyl substituents and nonsubstituted maleimides. Moreover, the electrondonating group, the methoxy (ether group) on the furan, increases the stability of the adduct with respect to methyl (alkyl group), while this substitution on electron poor maleimide exhibits an opposite effect. These substitutions, however are also predicted to decrease the transition state barriers, not hindering thus the reversibility of the reaction in spite of favoring the DA adduct formation. In accordance with these calculations, the applied maleimides show high conversions decreasing in the series (see
Figure 3);
αe(HBMI) >
αe(DPBMI) ~
αe(PPO3BMI).
The DA cross-linking of the partially broken down structure shows an important difference from the reaction of monomers both in solution and bulk. The reformation of the F4D2000–PPO3BMI network structure proceeds with a lower activation energy for the DA reaction,
Ea,DA = 9 kJ/mol, while the value for rDA reaction
Ea,rDA = 56 kJ/mol is in accordance with the solution data. The very low activation energy for the DA cross-linking reaction,
Ea,DA = 7.04 kJ/mol,
Ea,rDA = 57.9 kJ/mol, was reported also by Polgar et al. [
35]. They followed rheologically the reaction kinetics of cross-linking of EPM rubber with DA chemistry after previous decross-linking. The low activation energy was interpreted by diffusion limitation of the reaction in polymer systems. In contrast, Kuang et al. [
36] reported a higher
Ea of DA type cross-linking of SBR containing furan units due to more difficult diffusion of functional groups in the solid state reaction. We suppose that a low activation energy for the DA reaction is related to the particular topological arrangement of functional groups in the partially broken up structure. The unbroken bonds keep the local structure unchanged and the functionalities from the cleaved bonds in their vicinity are therefore still in a close contact and preserve their location before breaking. As a result, the reconnection of these adjacent functionalities, previously connected, is sterically facilitated and more likely than a random reaction with another functionality.
3.2. Network Formation
Formation of classical polymer networks, arising by stepwise alternating copolymerization reaction, is described by Flory–Stockmayer expression [
37] of the classical gelation theory:
Gelation of a polymer system occurs at a critical gel conversion αgel of monomers A and B. Under ideal random reaction, it depends only on functionality of monomers fA and fB, and a stoichiometry of composition r (= [B]/[A]). ([A] and [B] are concentrations of the corresponding functional groups.)
The network structure is characterized mainly by cross-linking density
ν, defined as concentration of elastically active chains, by fraction of the gel
wg and concentration of dangling chains. Usually, the cross-linking density is determined from elastic modulus in rubbery state according to the kinetic theory of rubber elasticity.
where
G is equilibrium shear modulus, R is the gas constant, and A is the front factor (A = 1 for affine networks and A = (
f − 2)/
f for phantom networks). It is assumed that a transition occurs from the phantom model, applied just beyond the gel-point, to the affine model valid for the fully cured network.
The theories of network formation describe structure evolution and gelation during a cross-linking polymerization. In addition to classical gelation theory [
37], the statistical theories, theory of branching processes [
38] or recursive method [
39] are used. We applied the theory of branching processes generating branched structures by combination of structural units in different reaction states, the distribution of which is determined from the reaction scheme (see
Supplementary Material). The theory predicts development of cross-linking density/modulus and a fraction of the gel as a function of conversion during formation of reversible networks. The reaction reversibility is taken into account by assuming the equilibrium conversion at any instant of the reaction.
3.3. DA Reversible Networks
The formation of dynamic DA thermoreversible networks is determined by thermodynamic equilibrium of the reversible reaction and its temperature dependence. Contrary to permanent networks, the gelation is governed also by temperature. In addition to the critical conversion at the gel-point, also the gel-point temperature Tgel for the sol–gel transition exists. This critical temperature depends on the gel-point conversion, being thus controlled by functionality of monomers and stoichiometry of composition, and moreover it is governed by temperature dependence of an equilibrium conversion of functional groups. We studied the networks with different functionality of the furan and maleimide monomers, both in the stoichiometric and off-stoichiometric composition, and with a different structure of bismaleimides showing different thermodynamic equilibrium and kinetics of the DA reaction. Moreover, the bismaleimide with a long poly(oxypropylene) chain, PPO30BMI, was also applied to prepare the DA network. This approach makes it possible to tune Tgel in a suitable temperature window and to get a better insight into the formation of reversible DA networks.
Based on the Flory–Stockmayer expression in Equation (2), the critical conversion of monomers
αgel = 0.58, in the stoichiometric tetrafuran–bismaleimide (F4-M2) networks. Due to the lower functionality of our tetrafuran (
fw = 3.84), the gel point conversion is 0.59.
Figure 3 displays the temperature dependence of equilibrium conversions of the maleimide functionality in the three F4-M2 systems differing in the maleimide structure; F4D2000–DPBMI, F4D2000–PPO3BMI, and F4D2000-HBMI. According to the figure, the following theoretical gel-point temperatures of the networks correspond to the theoretical critical conversion;
Tgel (F4-DPBMI) = 105 °C,
Tgel (F4-PPO3BMI) = 108 °C, and
Tgel(F4-HBMI) ~ 120 °C, respectively. Despite the equal functionality of the networks (F4-M2), and thus the equal
αgel in the ideal case, the theoretical
Tgel differ by 16 °C. This is a consequence of the maleimide monomer structure governing thermodynamics of the reversible DA reactions and thereby affecting formation of thermoreversible networks. The characterizations of the studied dynamic networks are summarized in
Table 1.
The thermoreversibility of networks was determined by rheology measurements and monitoring dynamic storage
G‘ and loss moduli
G“, as shown in
Figure 4. The specimen of a network was heated in the rheometer under plate/plate geometry up to 120 °C in order to decross-link the network. The
Figure 4a reveals that
G“ > G‘ at this temperature, implying the liquid character of the sample. The cooling of the melt (blue curves) by the rate 5 °C/min resulted in the network reformation by the cycloaddition DA reaction manifested by increase in modulus. The point of gelation was determined as a crossover of
G’(T) and
G“(T) curves (see
Section 2.4.2). The more precise gel-point evaluation corresponding to the Winter–Chambon criterion of the critical gel [
29] is discussed below. The system F4D2000–DPBMI in
Figure 4a gels at
Tgel = 86°C and modulus increases with decreasing temperature. The subsequent heating of the network (black curves) leads to a drop of shear modulus
G‘(T) at ~90 °C. The gel–sol transition, in this case, appears at a higher temperature: 108 °C (see
Table 1). The figure shows a very small difference (two dotted lines) in determination of the gel point by using the simplified method from the crossover of moduli compared to the evaluation from the critical value of the loss factor, (tan δ)
gel = 1.5, corresponding to the frequency independent loss factor (see Experimental).
The
Figure 4c,d reveal that the determined
Tgel is dependent on the experimental conditions, i.e. the cooling and heating rates, as it was previously proved [
3,
13,
15]. Gelation of F4D2000–DPBMI is shifted to a higher temperature at a slower cooling rate;
Tgel = 92 °C (rate of cooling 3 °C /min),
Tgel = 102 °C (1 °C /min),
Tgel = 107 °C (0.5 °C /min), which is in a good accordance with the theoretical value (see
Table 1). The delay of gelation at a fast cooling rate is a result of the nonequilibrium conditions during DA network formation. The DA reaction is too slow to reach an equilibrium state during a dynamic scan in a rheometer.
The effect of the cooling/heating rate on the DA reaction progress during the dynamic run is displayed in
Figure 5. The network F4D2000–DPBMI was partially broken down at 120 °C and the evolution of conversion during the subsequent cooling and heating processes was determined by FTIR. A short annealing time was applied at each temperature before determination of conversion, simulating thus a dynamic cooling/heating scan of the rate 3 °C /min. The figure illustrates nonequilibrium conditions and large differences of conversions from the equilibrium value (curve 3) determined after the 24 h annealing at the corresponding temperature. The difference is mainly significant during cooling (curve 1), i.e., during network formation. The critical gel-point conversion (
αgel = 0.58) is reached at a lower temperature, leading thus to a decrease in
Tgel with increasing cooling rate. Also the reverse gel–sol transition at heating (curve 2) due to the decrosslinking is delayed and the critical temperature for the gel–sol transition in this case is shifted to a higher value with respect to the equilibrium conditions. The difference from the equilibrium, however, is less significant in this case, and therefore also the dependence of
Tgel on heating rate is weaker, as it is obvious in
Figure 4d.
The relative rates of the DA reaction and an experimental procedure, i.e. the rate of cooling/heating (
vexp), play a role as an important kinetic parameter. The equilibrium conditions are achieved only in the case
vexp < vDA, vrDA. Otherwise, the dynamic measurement proceeds under the nonequilibrium state. According to the simulation of Scheltjens et al. and Diaz et al [
3,
15], the equilibrium at the temperature around
Tgel in their similar systems could never be met or only at very low cooling/heating rates (<0.5 °C /min). In our experiments, the equilibrium state and equilibrium temperatures
Tgel were determined by evaluating both (
Tgel)
cool and (
Tgel)
heat received at the cooling and heating scans, respectively (see
Table 1). By slowing the experimental rates these values approach from both sides to the equilibrium value. In the case of slow enough experimental scans (
Tgel)
cool = (
Tgel)
heat = (
Tgel)
equilibrium. For too slow DA reactions, the
Tgel was determined by interpolation (
Tgel)
cool <
Tgel < (
Tgel)
heat. An extreme example of nonequilibrium conditions was observed at formation of F4D2000–PPO30BMI network. Due to a large molecular weight of the bismaleimide PPO30BMI and a correspondingly low concentration of functional groups, the DA reaction is very slow. As a result, no gelation was observed during the dynamic cooling scan of the broken up structure even at a low cooling rate 1°C /min as illustrated in
Figure 4b. The experimental determination of gelation is thus affected by the kinetic effect apart from the thermodynamics.
Gelation of the F4D2000–HBMI system at cooling occurs in
Figure 4e already at a temperature
Tgel = 108 °C even at the high rate, 3 °C /min.
Table 1 shows that the equilibrium state is reached during the dynamic scan at the cooling/heating rate 0.5 °C/min. The determined equilibrium value
Tgel = 122 °C well agrees with the theoretical prediction. The network F4D2000–PPO3BMI, split up at 120 °C, undergoes gelation by cooling at
Tgel = 85 and 93 °C using the cooling rates 1 °C/min and 0.5 °C/min, respectively (see
Figure 4f).
Table 1 reveals, however, that the equilibrium was still not set at the rate 0.5 °C/min, because of the slow DA reaction as proved above. Due to a slow network reformation, the gelation was even not observed at the higher cooling rate, 3 °C/min. The equilibrium
Tgel = 97 °C was determined by interpolation in the region 93–100 °C (see
Table 1). This gel-point temperature is substantially lower than the theoretical prediction in
Table 1. In this case, a nonideality of the network formation has to be taken into account. The flexible bismaleimide PPO3BMI is prone to close cycles with the tetrafuran monomer F4D2000, in addition to the intermolecular bonding. The formation of intramolecular bonds at the expense of intermolecular ones does not contribute to a structure growth and a network build-up. It results thereby in delay of gelation and decrease in cross-linking density of a network. Consequently, the total conversion at the gel-point, involving both intermolecular and intramolecular bonds;
(αtotal)gel = (αinter)gel +
(αintra)gel, is increased in the F4D2000–PPO3BMI system, because only the intermolecular bonds are efficient in the network formation. As a result,
Tgel is shifted to a lower temperature with respect to the predicted theoretical value. Gelation at
Tgel = 97 °C corresponds to
(αtotal)gel = 0.65 (cf.
Figure 3) instead of the ideal value 0.59. One can assume that the extent of cyclization reaches the value
αintra(
= αtotal –αinter) = 0.06 at the gel-point.
In addition to the tetrafuran–bismaleimide (F4-M2) networks, the hexafunctional furan monomer F6T3000 and the trifunctional maleimide TMIEA were used. The increasing functionality of monomers leads to a decrease in the theoretical
αgel and increase in the
Tgel up to ~145 °C. The
Table 1 shows a relatively good agreement of the experimental and theoretical gel-point temperatures in the networks F6-M2 and F4-M3.
Another way of a gelation control is possible by varying the monomers composition. The off-stoichiometric networks (
r = [M]/[F] ≠ 1) show delay of gelation and form imperfect networks with dangling chains. The off-stoichiometric F4D2000–PPO3BMI network with maleimide in excess,
r = 1.5, exhibits the decrease in equilibrium
Tgel with respect to the stoichiometric network,
Tgel = 93 °C (see
Table 1).
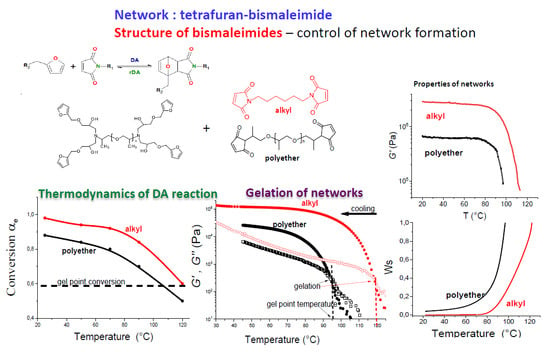
3.5. Modulus and Sol Fraction of DA Reversible Networks
In addition to the rate of gelation, the cross-linking density and the corresponding modulus, as well as the gel fraction are the main parameters characterizing a network formation. Due to the temperature-dependent conversion in thermoreversible networks, the cross-linking density and the gel fraction are functions of temperature and the modified expression holds for the equilibrium modulus:
The G‘/T plots of the homogeneous networks F4D2000–PPO3BMI, F4D2000–PPO30BMI and F4D2000-HBMI are illustrated in
Figure 10a. Moreover, the corresponding theoretical curves are included as dash lines. For the theoretical calculation, the equilibrium conversion is taken at any temperature. At increasing temperature, the theoretical equilibrium moduli decrease due to the diminishing conversion of the DA reaction. In contrast, the experimental moduli do not decline or only slightly until 70 °C. The reversible networks are in equilibrium state at ambient temperature, however, at higher temperatures the conditions are nonequilibrium during the temperature sweep. The DA/rDA reactions are slow in comparison to the rate of heating (2 °C/min) and the conversion does not reach the equilibrium value. Two experimental equilibrium points at higher temperatures, received by isothermal annealing of F4D2000–PPO30BMI, are included in the figure for a comparison with the theory.
The experimental moduli are in agreement with the theoretical ones for PPO30 containing network and in a relatively good accordance for the „HBMI“ network. The lower value of the experimental modulus in the „PPO3“ network is brought about by cyclization as discussed above. The agreement of the equilibrium modulus with the theory at room temperature is achieved assuming the intramolecular reaction in the extent αintra = 0.04 (curve 1‘c).
Consequently, four independent results provide an evidence of cyclization in the PPO3BMI containing network. The fraction of intramolecular bonds was determined to be in the range 0.04-0.12 from the shift of
Tgel, from the critical
αgel value at isothermal network formation, from the postgel growth of modulus, and from the equilibrium modulus of the cured network at room temperature. No cyclization occurs in the „PPO30BMI“ network because the corresponding ring in this case is too large. The relative tendency to cyclization wih respect to the intermolecular reaction depends on the chain flexibility and the size of the smallest possible cycle. With increasing size of this ring the cyclization probability significantly decreases [
40]. Neither HBMI network likely undergoes cyclization. There is a phase separation of the aliphatic bismaleimide and the propylenoxide based furan monomer at the early stages of the reaction (see
Supplementary Materials, Figure S11), and therefore the intermolecular reaction is preferred with respect to the intramolecular one. The cyclization with the rigid DPBMI molecule is also unlikely.
The reversible networks show a high sol fraction, which limits their materials applicability. The equilibrium values
wS (
=1 − wg) cannot be determined experimentally, because an extraction of the monomers and sol fraction from the network leads to a continuous shift of the reversible DA reaction towards monomers. The F4D2000–PPO3BMI network completely dissolves in DMSO at 50 °C, despite being far below
Tgel. The problem of the sol fraction in reversible networks is not investigated in literature, despite being extremely important for a materials application. Taking into account the reasonable agreement of the theory with experimental moduli, one also assumes that w
S values could be well predicted. The theory was used to provide the information on this important aspect. The theoretical w
S as a function of temperature are shown in
Figure 10b. In the case of the „HBMI“ network, the sol fraction is negligible up to 80 °C. However, the PPO3BMI containing network involves at 80 °C already a quite high fraction of the sol (>0.20) due to the lower equilibrium conversion and the cyclization. The effect of the DA reaction thermodynamics is revealed by comparison of the curves 1 and 2 for PPO3BMI and HBMI containing networks, and the influence of cyclization is displayed by the curve 1‘, characterizing „PPO3BMI“ network undergoing 6 % of intramolecular reactions.
3.6. Dynamics of the Transient Networks
The thermoreversible networks exhibit a transient character as proved by the rheology frequency sweeps of the networks F4D2000–DPBMI and F4D2000–HBMI shown in
Figure 11.
Three regions are observed in the curves of the storage modulus as a function of frequency in the F4D2000–DPBMI network (
Figure 11a); a wide region of the rubbery plateau, a flow region at a low frequency, and vitrification at a high frequency. This network exhibits a relatively high glass transition temperature,
Tg = 40 °C, and therefore at 50 °C and high frequencies, the modulus is affected by the glass transition.
The networks show a rubbery modulus plateau with the storage modulus
G‘ independent of frequency at temperatures
T < Tgel, where
G‘ > G“. The plateau modulus decreases with increasing temperature due to diminution of the conversion. In the network containing DPBMI, the modulus plateau is observed at
T = 95 °C, however, at 100 °C, the plateau disappears and the moduli
G’(ω) and
G“( ω) show the similar scaling at higher frequencies,
G‘(ω)~G“(ω)~ωn (n = 0.64). This state corresponds to the Winter–Chambon criterion for the gel-point [
29]. The critical gel occurs in the temperature region 100–105 °C, which is in a good agreement with the
Tgel equilibrium value determined by the simplified method from the crossover of
G’and
G“ measured at a constant frequency (see
Figure 4,
Table 1). At a higher temperature,
T = 110 °C, the polymer exhibits liquid-like behavior;
G“ > G‘,
G‘~ω2, G“~ ω1. While the networks with DPBMI a PPO3BMI maleimides show a similar dynamic behavior, the „HBMI“ network exhibits a higher thermal stability. The broad plateau, characterizing the solid network state, exists even at 110 °C and only at
T = 120 °C the polymer is approaching the critical state and flows.
The liquid-like behavior appears, at the low-frequency, even at temperatures well below
Tgel, i.e. far above the gel-point conversion. The terminal relaxation of the network is a macroscopic demonstration of a network decross-linking by the rDA reaction. The relaxation time of the network
τr was determined from the frequency crossover
ωC (τr = 2π/ωC) in
Figure 11. The crossover of
G’(
ω) and
G”(
ω) is gradually shifted to a higher frequency at increasing temperature, i.e., the relaxation time
τr becomes shorter, revealing thus the reducing stability of the network. The network connectivity is thus broken at shorter times when approaching to
Tgel. Also this characterization of the network dynamics reveals the highest stability of the HBMI containing networks. The relaxation times are similar in the case of „DPBMI“ and „PPO3BMI“ networks, but they are much longer for the „HBMI network“. At 90 °C the following values were determined;
τr (DPBMI) = 400 s and
τr (HBMI) > 2000s. Even at 100 °C and 110 °C the HBMI network is relatively stable with
τr = 1000 s and 170s, respectively.
The dynamics of the DA networks thus involves the thermodynamically governed network breaking at higher temperatures due to the temperature dependence of the equilibrium conversion. Moreover, there is the kinetically controlled time dependent network disconnection at any temperature related to lifetime of the reversible bond. At a high temperature, the dynamic character becomes more pronounced because of higher rates of the bond forming/breaking reactions and a shorter bond lifetime. The relative rates of the DA reaction and experimental dynamic measurements govern the time scale of the transient region from an elastic network behavior to the liquid state. At low temperatures (50 °C in DPBMI and 90 °C in HBMI), the reaction rates are relatively too small, the networks are more stable and the transition is shifted to long time scales to be observed during the experimental measurement conditions.
3.7. Design of a Self-healing Network
The understanding of the reversible networks formation is a basic step to optimize the self-healing procedure and to design a proper structure of a self-healing network. For an efficient and promising self-healing, the polymer network should be healed in a suitable temperature window and the healing, including structure reformation, should be rapid. Moreover, the incomplete conversion in the reversible networks results in a reduction of modulus and a high fraction of the sol even at ambient temperature. The corresponding poor mechanical properties and leaching of monomers present the significant drawback of the reversible networks. Therefore, the high stability of the network at ambient temperature is desirable.
The DA network breaking/formation is affected by morphology of a system and its physical state. In order to design the system for self-healing at an appropriate region of temperatures (20–130 °C), the glass transition temperature
Tg and gel-point temperature
Tgel of the network must be tuned by controlling the monomers structure and functionality. Low enough
Tgel (<130 °C) prevents participation of irreversible side reactions and low
Tg (<50 °C) excludes vitrification and a significant deceleration of the DA reaction in a network. The optimum system exhibits a rubbery modulus plateau in the proper temperature range providing enough space for a structure reconstruction at cooling. The applicability of the studied networks is obvious from DMTA plots in
Figure 12, characterizing roughly their morphology. The F4D2000–PPO3BMI and F4D2000–PPO30BMI networks are homogeneous and rubbery at ambient temperature. Their structure evolution could be well described by theory and thereby they serve as ideal systems to get an insight into the DA network formation. Also HBMI containing network and the heterogeneous network involving DPBMI show a rubbery plateau despite a relatively high
Tg in the „DPBMI“ network shortening the applicable rubbery plateau region. In contrast, the networks from monomers of a higher functionality: F6T3000-DPBMI (F6-M2) and F4D2000-TMIEA (F4-M3) exhibit too high
Tgel (>130 °C). The network F3FGEFA-PPO3BMI, containing the short trifuran monomer instead of the long flexible F4D2000, is also unsuitable due to the high
Tg preventing thus a DA network reformation because of vitrification.
The network F4D2000-HBMI was shown to be the best system from the point of the self-healing view. The alkyl substituent in the maleimide increases stability of the DA adduct, while it does not prevent reversibility of the reaction. This dynamic network is very stable at ambient temperature showing the conversion
αe > 0.95, and therefore it exhibits a relatively high modulus and a negligible sol fraction,
wS < 0.01(see
Figure 10b). It also undergoes a very rapid structure healing as determined by simulating the healing procedure and following network reformation from a broken structure (see
Figure 9c).