3.1. Differential Scanning Calorimetry (DSC)
The non-isothermal DSC curves at a heating rate of 5 K/min for the epoxy-thiol system without any filler (ETL) and for the epoxy-thiol-BN composite systems filled with 2, 30 and 180 µm BN platelets are shown in
Figure 2, while similar results were obtained for heating rates of 2 and 10 K/min. These DSC scans were made from −60 °C, but only the temperature region in which the exothermic cure reaction occurs is shown here. At lower temperatures, however, the glass transition temperatures of the uncured mixtures,
Tg0, with 2, 30 and 180 µm BN platelets are observed at the average temperatures (±1 standard deviation) of −38.6 ± 0.5, −38.0 ± 0.7 and −37.5 ± 0.7 °C, respectively, compared with −38.4 ± 0.5 °C for the unfilled ETL system. The glass transition temperature of the uncured mixture is therefore essentially independent of the heating rate and of the BN content; the small increase in
Tg0 with increasing BN particle size may be a result of an increased restriction to the molecular mobility of the epoxy-thiol mixture with the larger particles, and is of no further consequence here.
In a second scan after the non-isothermal cure shown in
Figure 2, the glass transition temperature of the fully cured system,
Tg∞, is determined, and is found to be approximately independent of BN particle size and content, with values of 54.0 ± 0.4, 53.7 ± 1.2 and 52.7 ± 1.3 °C for the 2, 30 and 180 µm BN platelets, respectively. Likewise, the heat of reaction, Δ
H, is also found to be essentially independent of BN particle size and content, as well as of the heating rate, with an average value of 127.2 ± 8.3 kJ/ee. The constancy of both
Tg∞ and Δ
H implies that the network structure of the cured epoxy composites is not influenced by the presence of the BN filler.
On the other hand, the kinetics of the cure reaction clearly is influenced by the BN filler, as can be seen from two particular aspects of the cure curves in
Figure 2. The first aspect is the displacement of the curves as a function of BN content. The addition of BN particles results in a shift of the curves to higher temperatures. This is consistently observed for all BN platelet sizes, but is most dramatic for the smallest particle size of 2 μm, for which the exothermic peak is shifted by as much as 30 °C. The explanation for this observation probably lies in the relationship between surface area and volume of the BN platelets. If, as we argue here, there is an interaction between the epoxy-thiol matrix and the BN filler, which is a surface effect, then the surface area to volume ratio will have an important influence. In fact, it is probably only the edge surfaces of the platelets which are involved in this interaction, for which the area to volume ratio is inversely proportional to the particle size. Furthermore, the 180 µm platelets have a much wider distribution of particle sizes than do the other platelet sizes (see
Figure 1), and there is also a tendency for the larger platelet sizes to break during fabrication, evidenced by the fracture surfaces of the cured composites (to be discussed below). Consequently, the area-to-volume ratio for the 30 and 180 µm platelets is rather similar, whereas that for the 2 µm platelets is an order of magnitude larger.
The second aspect is that the cure curves become increasingly asymmetric as the BN content increases, this being particularly noticeable in
Figure 2 for the composites filled with 30 and 180 μm platelets. For the composites filled with the 2 µm platelets, on the other hand, this asymmetry takes the appearance of a shoulder at low temperatures, which becomes more prominent as the BN content increases, eventually even being manifest as a small peak for the highest BN content. The explanation for this behavior probably lies in the action of the LC-80 initiator, which is an encapsulated imidazole. This encapsulation means that, in principle, the initiation of the epoxy-thiol reaction by the LC-80 occurs when the temperature of 70 to 80 °C is attained during non-isothermal cure. In addition, though, the reaction kinetics is influenced also by the presence of the BN platelets, and in a different way depending on the platelet size, as discussed immediately above. The consequence of this combination of effects is the different asymmetry observed in
Figure 2 for the 2 µm platelets in comparison with that observed for the 30 and 180 µm platelets.
The shift of the peaks to higher temperatures with increasing BN content can be seen more readily in
Figure 3, where the peak exothermic temperature,
Tp, relative to that for the unfilled system,
Tp0, is plotted as a function of the vol % of BN for each particle size and for each heating rate. The temperature shift is clearly greatest for the 2 µm platelets, and then reduces in turn for the 30 and 180 µm particles.
For all particle sizes, it is interesting to observe that there is no significant difference between the shifts for the two lower heating rates of 2 and 5 K/min. At first sight, this may appear strange, but it must be remembered that it is the
difference between the peak temperatures of the filled and unfilled systems that is plotted in
Figure 3; in all cases, the peak temperature itself always increases with increasing heating rate, as would be expected. What the results for the heating rates of 2 and 5 K/min probably indicate is that the cure reaction is not simple, as has already been commented in respect of the results presented in
Figure 2, and that there may be competing effects of the BN filler particles. In particular, there appears to be a tendency, especially for the 30 µm composites, for the reaction first to be accelerated on the addition of BN particles, before being slowed.
Similar effects are observed also for the isothermal cure.
Figure 4 shows the isothermal cure at 70 °C of samples with different contents of the three BN particle sizes: ETLBN2, ETLBN30 and ETLBN180. For each cure condition, the heat of reaction (122.8 ± 9.6 kJ/ee) and the glass transition temperature of the fully cured sample (52.7 ± 0.8 for ETLBN2, 53.0 ± 0.9 for ETLBN30, 51.8 ± 1.3 °C for ETLBN180), obtained from a second scan, are essentially independent of the filler content and of the isothermal cure temperature. On the other hand, the cure kinetics is significantly influenced by the BN content. In general, with increasing amount of filler, whatever the size of the platelets, the exothermic peak is displaced to longer times, similar to the displacement to higher peak temperatures in the non-isothermal cure curves in
Figure 2. However, for the smallest BN content (
y=10), the cure is actually accelerated for the 2 and 180 μm platelets, an effect that can be observed also in some of the non-isothermal results, for example for the 30 µm platelets, shown in
Figure 3. Similar results were found for the other isothermal cure temperatures of 60 and 80 °C.
The variation of the peak exotherm time,
tp, with the BN content is shown in
Figure 5 for the three BN platelet sizes and for the three isothermal cure temperatures. Here can be seen the tendency for the reaction first to accelerate, with a reduction in
tp, and then to be slowed as the BN content increases. There is a systematic variation with the BN platelet size, whereby the peak time
tp, for a given cure temperature and BN content, decreases as the BN platelet size increases, and dramatically so on going from 2 µm to 30 μm; this behavior mirrors that shown in
Figure 3 for the non-isothermal cure.
In order to understand the role of the thiol cross-linking agent in the kinetics of the cure reaction, it is of interest to compare these results for the cure of these epoxy-BN composites with a thiol with those for the same epoxy-BN system cured with a diamine, Jeffamine D-420. This comparison has been made for just one of the BN platelet sizes (30 µm). The corresponding results for the non-isothermal cure of the EJBN30-
y samples at 2 and at 5 K/min are shown together with the results for the ETLBN30-
y samples at the same heating rates in
Figure 6. It is evident that the peak temperature for the epoxy-diamine system is essentially independent of the BN filler content, in clear contrast to the behavior for the epoxy-thiol system. This was also reported earlier in the comparison of epoxy-thiol and epoxy-diamine systems filled with aluminium nitride, AlN [
7].
There is clearly an influence of the BN filler on the cure kinetics of the epoxy-thiol system, though the fully cured epoxy network structure is unaffected, and this effect is related to the presence of the thiol as it is not observed for the epoxy-diamine system. In this context, the effect of the BN particle size is important.
Figure 7 shows how the peak exotherm temperature in non-isothermal cure at 5 K/min depends on the platelet size. It can be seen that the greatest effect of retarding the reaction occurs for the smallest platelets, while there is very little difference between the effects of the 30 and 180 µm platelets. This behavior occurs also for the heating rates of 2 and 10 K/min, and the systematic effect of the particle size and of the filler content has been noted in earlier work [
7,
8].
Since this interaction between the epoxy matrix and the BN filler is fundamental in respect of the heat transfer by phonon transport in the composite material, this systematic behavior should be compared with the thermal conductivities of these epoxy-thiol composites filled with BN platelets of different sizes. First, however, it is of interest to consider the composition of the cured composites by means of thermogravimetric and density measurements.
3.2. Thermogravimetric Analysis (TGA)
The thermal degradation of the cured systems has been determined by TGA under an inert atmosphere of dry nitrogen, and the weight loss at 10 K/min for the composites with the 30 μm BN platelets is shown in
Figure 8; similar results were obtained for the composite samples with 2 and with 180 μm platelets, and for the heating rate of 2 K/min. In addition, the degradation behavior of the epoxy-thiol system without any filler (ETL) was also determined, and that of the BN particles alone were also studied by TGA, though these results are not shown in
Figure 8. The degradation behavior is very similar for the composites fabricated with BN platelets of different sizes, as would be expected from our earlier observation that the epoxy network structure is independent of the BN platelet size and content. For each platelet size, the important difference between the various curves is the residual mass at 600 °C, which increases as the BN content increases.
The cured epoxy-thiol without any filler, ETL, leaves a residue of 4.5 ± 2.5%, while the BN particles alone leave a residue of 97 ± 2%. Taking a 60:40 mass ratio for the stoichiometric epoxy:thiol ratio, with 2 wt % LC-80 initiator, then the anticipated residues for the different BN contents can be calculated as: 10.2 ± 2.5% for ETLBN
x-10; 23.2 ± 2.4% for ETLBN
x-30; 38.9 ± 2.3% for ETLBN
x-50; 48.0 ± 2.2% for ETLBN
x-60; 58.2 ± 2.2% for ETLBN
x-70, independently of the size,
x, of the platelets. The average values obtained by TGA for the two different heating rates of 2 and 10 K/min are plotted as a function of these theoretical values in
Figure 9. It can be seen that the correlation is very good, confirming thus the BN contents of the different composite samples as well as supporting the conclusion that the epoxy network structure is independent of the filler size and content.
3.4. Thermal Conductivity
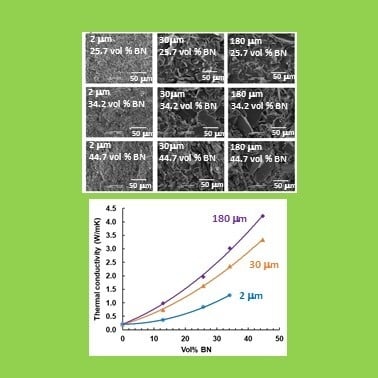
The dependence of thermal conductivity on BN content is shown in
Figure 11. As expected, and as has been observed in the large majority of studies of such systems [reference 8 gives a compilation], the thermal conductivity increases with BN content, and more so the greater is the BN content, giving an upward curvature to the dependence. The highest vol % of 44.7% represents the approximate limit that can be achieved with the simple mixing procedures adopted here, as the mixture becomes a very stiff paste at these BN contents, and is intractable for higher concentrations. Indeed, the vol % of 44.7% cannot be achieved for the samples with 2 μm platelets, as explained in an earlier section.
It can also be seen very clearly that the thermal conductivity increases systematically with increasing BN platelet size. In this study, we have not made use of any surface treatment of the BN particles or of any coupling agent to improve the contact between particles and matrix. Furthermore, the BN particles all have the same shape (platelets), as well as all being from the same supplier (Saint Gobain). There can therefore be no doubt that increasing BN particle size results in an increase in the thermal conductivity when composites with the same filler content are compared. This confirms what the majority of studies have suggested, but which until now has been unclear as a consequence of the possible influence of the other parameters, which have been eliminated in the present study.
The increase of thermal conductivity with BN particle size can be understood from a consideration of the interface between particles and matrix, and of the geometry of the particles. In view of the very high value of thermal conductivity of hexagonal BN (600 W/mK parallel to the basal plane; 30 W/mK perpendicular to the basal plane), the fact that more limited values are obtained for epoxy-BN composites in general is evidently in part a consequence of the interface between particles and matrix, which presents a resistance to heat flow. Indeed, this is the reason why coupling agents and surface treatments are often introduced into the composite preparation procedure, though not always with a significant improvement in the thermal conductivity. For a given BN content, the larger is the particle size, the smaller is the interfacial area, and hence increased particle size is clearly beneficial. However, if the platelets are considered to be hexagonal with edge size d/2 and thickness t, then the ratio of area:volume is A/V = 2/t + 8/d√3. With t = 0.2 μm this gives values of A/V of 12.3, 10.2 and 10.0 μm−1 for the 2, 30 and 180 μm platelets, respectively; with t = 1.0 μm the corresponding values of A/V are 4.3, 2.2 and 2.0. The increase of 20 to 30% in the thermal conductivity between the samples with 30 and 180 μm platelets is difficult to reconcile with the much smaller increase in the corresponding area:volume ratio.
It is in this respect that the geometry of the particles is believed to play an important part, and in particular the anisotropy of the thermal conductivity. The larger platelets provide significantly more continuity in the parallel direction, with a thermal conductivity of 600 W/mK, than do the smaller platelets, and this additional factor results in the highest thermal conductivities of the 180 μm composites.
In fact, these composites with BN platelets of 180 μm, and also those with 30 µm platelets to a certain extent attain values that are significantly larger than most published values for the same BN content [
8]. We believe that the reason for this is associated with the improved interface between matrix and particles as a consequence of the use of thiol as the cross-linking agent. This improvement results from the Lewis acid-base interaction between the sulphur of the thiol and the boron of the particles. In order to investigate this interpretation, epoxy-BN composite samples with the 30 µm platelets (EJBN30-
y) were prepared in which the cross-linking agent was a diamine rather than the thiol. The resulting thermal conductivities as a function of the BN content are included in
Figure 11, where it can be seen that they fall significantly below the corresponding values for the epoxy-BN composites cross-linked with thiol. The explanation for this is the matrix-filler interface is enhanced when the sulphur-containing thiol is used.
This distinction between the behaviors of the epoxy-BN composite system filled with 30 µm BN platelets and cured with thiol on the one hand and with diamine on the other was seen earlier in the cure kinetics monitored by DSC, shown in
Figure 6. For the composites cured with thiol there is a strong and systematic dependence of the peak temperature on BN content, whereas for the diamine the peak temperature is essentially independent of the filler content. This confirms that there is an interaction between the epoxy-thiol matrix and the BN particles which influences both the cure kinetics and the matrix-filler interface, this latter leading to an increased thermal conductivity, whereas there is no such interaction when epoxy-diamine is used for the matrix. None of the results published by other authors and discussed here made use of thiol as a cross-linking agent, and we attribute the higher thermal conductivity values reported here to the advantageous interactions between the thiol and the filler particles.