Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles
Abstract
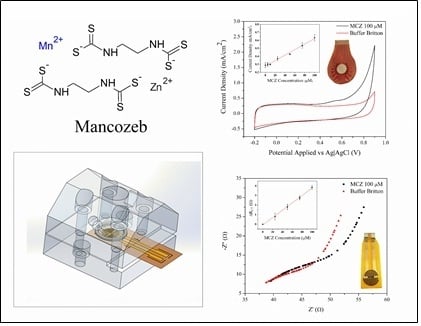
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mancozeb Thermal Characterization
2.3. Electrode Fabrication and Characterization
2.4. Preparation and Degradation of Mancozeb in Solution
2.4.1. Preparation of Mancozeb Solutions
2.4.2. Thermal Degradation of Mancozeb in Solution
2.4.3. Photodegradation of Mancozeb in Solution under UV Light in a Photoreactor System
2.4.4. Electrochemical Degradation of Mancozeb in Solution under Galvanostatic Conditions
2.4.5. Quantification of the Mancozeb in Solution
3. Results and Discussion
3.1. Physicochemical Characterization of the Monomer and Nanoparticles
3.2. Thermogravimetric Characterization of Mancozeb
3.3. Quantification of Mancozeb in Solution
3.4. Temperature and Time in the Mancozeb Degradation
3.5. Effect of UV Irradiation
3.6. Degradation of Mancozeb
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Echeverría-Sáenz, S.; Mena, F.; Pinnock, M.; Ruepert, C.; Solano, K.; de la Cruz, E.; Campos, B.; Sánchez-Avila, J.; Lacorte, S.; Barata, C. Environmental hazards of pesticides from pineapple crop production in the Río Jiménez watershed (Caribbean Coast, Costa Rica). Sci. Total Environ. 2012, 440, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, S.; Csomor, J.; Urbanek, P.; Pelclova, D. Case Report Toxic Epidermal Necrolysis After Exposure to Dithiocarbamate Fungicide Mancozeb. Basic Clin. Pharmacol. Toxicol. 2016, 118, 87–91. [Google Scholar]
- Iorio, R.; Castellucci, A.; Rossi, G.; Cinque, B.; Cifone, M.G.; Macchiarelli, G.; Cecconi, S. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol. Vitr. 2015, 30, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb: Past, Present, and Future. Plant Dis. 2010, 94, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Kong, Y.H.; Wu, C.Y.; Yin, C.M.; Wang, G.H.; Liu, Z.R. The thermal behaviour of the ethylenebis-dithiocarbamates Maneb, Zineb and Mancozeb: Part 3. Thermal stability of Maneb, Zineb and Mancozeb. Thermochim. Acta 1993, 220, 237–244. [Google Scholar] [CrossRef]
- Hwang, E.S.; Cash, J.N.; Zabik, M.J. Determination of degradation products and pathways of mancozeb and ethylenethiourea (ETU) in solutions due to ozone and chlorine dioxide treatments. J. Agric. Food Chem. 2003, 51, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Van Wendel de Joode, B.; Mora, A.M.; Córdoba, L.; Cano, J.C.; Quesada, R.; Faniband, M.; Wesseling, C.; Ruepert, C.; Öberg, M.; Eskenazi, B.; et al. Aerial application of mancozeb and urinary ethylene thiourea (ETU) concentrations among pregnant women in Costa Rica: the Infants’ Environmental Health Study (ISA). Environ. Health Perspect. 2014, 122, 1321–1328. [Google Scholar] [CrossRef]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef]
- López-Fernández, O.; Yáñez, R.; Rial-Otero, R.; Simal-Gándara, J. Kinetic modelling of mancozeb hydrolysis and photolysis to ethylenethiourea and other by-products in water. Water Res. 2016, 102, 561–571. [Google Scholar] [CrossRef]
- López-Fernández, O.; Pose-Juan, E.; Rial-Otero, R.; Simal-Gándara, J. Effects of hydrochemistry variables on the half-life of mancozeb and on the hazard index associated to the sum of mancozeb and ethylenethiourea. Environ. Res. 2017, 154, 253–260. [Google Scholar] [CrossRef]
- López-Fernández, O.; Barroso, M.F.; Fernandes, D.M.; Rial-Otero, R.; Simal-Gándara, J.; Morais, S.; Nouws, H.P.A.; Freire, C.; Delerue-Matos, C. Voltammetric analysis of mancozeb and its degradation product ethylenethiourea. J. Electroanal. Chem. 2015, 758, 54–58. [Google Scholar] [CrossRef]
- Bottrel, S.E.C.; Amorim, C.C.; Leão, M.M.D.; Costa, E.P.; Lacerda, I.A. Degradation of ethylenethiourea pesticide metabolite from water by photocatalytic processes. J. Environ. Sci. Heal. 2014, 49, 263–270. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Brunner, A.M.; Vughs, D.; Siegers, W.; Bertelkamp, C.; Hofman-Caris, R.; Kolkman, A.; ter Laak, T. Monitoring transformation product formation in the drinking water treatments rapid sand filtration and ozonation. Chemosphere 2019, 214, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Mayer, B.K.; Johnson, C.; Yang, Y.; Wellenstein, N.; Maher, E.; McNamara, P.J. From micro to macro-contaminants: The impact of low-energy titanium dioxide photocatalysis followed by filtration on the mitigation of drinking water organics. Chemosphere 2019, 111–121. [Google Scholar] [CrossRef]
- Masschelein, W.J.; Rice, R.G. Ultraviolet Light in Water and Wastewater Sanitation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429143199-CAT# KE83669. [Google Scholar]
- Rozas, O.; Vidal, C.; Baeza, C.; Jardim, W.F.; Rossner, A.; Mansilla, H.D. Organic micropollutants (OMPs) in natural waters: Oxidation by UV/H2O2 treatment and toxicity assessment. Water Res. 2016, 98, 109–118. [Google Scholar] [CrossRef]
- Hermes, N.; Jewell, K.S.; Wick, A.; Ternes, T.A. Quantification of more than 150 micropollutants including transformation products in aqueous samples by liquid chromatography-tandem mass spectrometry using scheduled multiple reaction monitoring. J. Chromatogr. A 2018, 1531, 64–73. [Google Scholar] [CrossRef]
- Schappler, J.; Guillarme, D. Current and future trends in UHPLC. TrAC Trends Anal. Chem. 2014, 63, 2–13. [Google Scholar]
- Sun, Q.; Dong, M.; Wen, G.; Li, Y.; Song, W.; Tang, H.; Wang, W. A novel approach for simultaneous determination of E/Z-fluoxastrobins in vegetables and fruits by UHPLC-DAD. Food Control 2017, 78, 7–13. [Google Scholar] [CrossRef]
- Walter, T.H.; Andrews, R.W. Recent innovations in UHPLC columns and instrumentation. TrAC Trends Anal. Chem. 2014, 63, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, R.P.; Oliveira, F.A.S.; Madureira, F.D.; Silva, G.; de Souza, W.R.; Lopes, R.P. Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC-MS/MS analysis. Food Control 2013, 33, 413–423. [Google Scholar] [CrossRef]
- Zamora, R.; Masís-Meléndez, F.; Phillips, H.; Alvarado-Marchena, L.A.; Starbird, R. Development of Poly(3,4-ethylenedioxythiophene(PEDOT)/carbon nanotube electrodes for electrochemical detection of Mancozeb in water. Int. J. Electrochem. Sci. 2018, 13, 1931–1944. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Zoski, C. Handbook of Electrochemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-51958-0. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; ISBN 0-471-04372-9. [Google Scholar]
- Kamin, R.A.; Wilson, G.S. Rotating Ring-Disk Enzyme Electrode for Biocatalysis Kinetic Studies and Characterization of the Immobilized Enzyme Layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Lee, C.; Lai, K.; Lin, C.; Li, C.; Ho, K.; Wu, C.; Lau, S.; He, J. A paper-based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 2017, 36, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Taroni, P.J.; Santagiuliana, G.; Wan, K.; Calado, P.; Qiu, M.; Zhang, H.; Pugno, N.M.; Palma, M.; Stingelin-stutzman, N.; Heeney, M.; et al. Toward Stretchable Self-Powered Sensors Based on the Thermoelectric Response of PEDOT: PSS / Polyurethane Blends. Adv. Funct. Mater. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Konwer, S.; Begum, A.; Bordoloi, S.; Boruah, R. Expanded graphene-oxide encapsulated polyaniline composites as sensing material for volatile organic compounds. J. Polym. Res. 2017, 24, 37. [Google Scholar] [CrossRef]
- Pumera, M. Nanomaterials for Electrochemical Sensing and Biosensing; Pan Stanford Publishing Pte. Ltd.: Singapore, 2014; ISBN 978-981-4364-90-4. [Google Scholar]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Spain, E.; Keyes, T.E.; Forster, R.J. Biosensors and Bioelectronics DNA sensor based on vapour polymerised pedot films functionalised with gold nanoparticles. Biosens. Bioelectron. 2013, 41, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhong, J.; Waqas, M.; Jiang, Z.; Fan, Y.; Sun, Y.; Li, J.; Chen, W. Controllable synthesis of six corner star-like Cu 2 O / PEDOT-MWCNT composites and their performance toward electrochemical glucose sensing. Electrochim. Acta 2019, 318, 837–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, T.; Wan, Y.; Chen, S. Gold nanoparticles-carbon nanotubes modified sensor for electrochemical determination of organophosphate pesticides. Microchim. Acta 2009, 165, 307–311. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Wang, H.; Hsu, J.H.; Yi, S.I.; Kim, S.L.; Choi, K.; Yang, G.; Yu, C. Thermally Driven Large N-Type Voltage Responses from Hybrids of Carbon Nanotubes and Poly(3,4-ethylenedioxythiophene) with Tetrakis(dimethylamino)ethylene. Adv. Mater. 2015, 27, 6855–6861. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.J.; Yambem, S.D.; Johns, A.H.; Afre, R.A.; Ellis, A.V.; Shapter, J.G.; Andersson, G.G.; Quinton, J.S.; Burn, P.L.; Meredith, P.; et al. Planar silver nanowire, carbon nanotube and PEDOT:PSS nanocomposite transparent electrodes. Sci. Technol. Adv. Mater. 2015, 16, 025002. [Google Scholar] [CrossRef]
- Starbird, R.; Bauhofer, W.; Meza-Cuevas, M.; Krautschneider, W.H. Effect of experimental factors on the properties of PEDOT-NaPSS galvanostatically deposited from an aqueous micellar media for invasive electrodes. In Proceedings of the 5th 2012 Biomedical Engineering International Conference, Ubon Ratchathani, Thailand, 5–7 December 2012. [Google Scholar]
- Tian, F.; Casey, A.; Shanahan, A.; Byrne, H.; Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface Enhanced Raman Scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 22, 9116–9123. [Google Scholar] [CrossRef]
- Wang, B.; Akiba, U.; Anzai, J.I. Recent progress in nanomaterial-based electrochemical biosensors for cancer biomarkers: A review. Molecules 2017, 22, 1084. [Google Scholar] [CrossRef]
- Giroud, N.; Dorge, S.; Trouvé, G. Mechanism of thermal decomposition of a pesticide for safety concerns: Case of Mancozeb. J. Hazard. Mater. 2010, 184, 6–15. [Google Scholar] [CrossRef]
- Talrose, V.; Yermakov, A.N.; Usov, A.A.; Goncharova, A.A.; Leskin, A.N.; Messineva, N.A.; Trusova, N.V. UV/Visible Spectra-NIST Chemistry WebBook. Natl. Inst. Stand. Technol. 2018, 69. [Google Scholar] [CrossRef]
- Petha, N.H.; Lokhande, R.S.; Seshadri, D.T.; Patil, R.M.; Bhagat, T.S.; Patil, J.G. A simple pre-column derivatization method for the determination of mancozeb technical (fungicide) by reverse phase HPLC-UV. Anal. Methods 2017, 9, 4702–4708. [Google Scholar] [CrossRef]
- Hernandez-Suarez, P.; Ramírez, K.; Alvarado, F.; Avendano, E.; Starbird, R. Electrochemical characterization of poly(3,4-ethylenedioxythiophene)/ κ-carrageenan as a biocompatible conductive coat for biologic applications. MRS Commun. 2018, 9, 218–223. [Google Scholar] [CrossRef]
- Calumpang, S.M.F.; Medina, M.J.B.; Roxas, N.P.; Magallona, E.D. International Journal of Pest Management Movement and degradation of mancozeb fungicide and its metabolites, ethylenethiourea and ethyleneurea in silty clay loam soil. Int. J. Pest Manag. 1993, 39, 161–166. [Google Scholar] [CrossRef]
- Montero-Rodríguez, J.J.; Schroeder, D.; Krautschneider, W.; Starbird, R. Equivalent circuit models for electrochemical impedance spectroscopy of PEDOT-coated electrodes. In Proceedings of the 6th IEEE Germany Student Conference, Hamburg, Germany, 28 September–3 October 2015. [Google Scholar]
- Shamsipur, M.; Kazemi, S.H.; Mousavi, M.F. Impedance studies of a nano-structured conducting polymer and its application to the design of reliable scaffolds for impedimetric biosensors. Biosens. Bioelectron. 2008, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ekman, E.; Maxe, M.; Littorin, M.; Jönsson, B.A.G.; Lindh, C.H. High-throughput method for the analysis of ethylenethiourea with direct injection of hydrolysed urine using online on-column extraction liquid chromatography and triple quadrupole mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 934, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-alam, J.; Bom, L.; Chbani, A.; Fajloun, Z.; Millet, M. Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J. Chromatogr. Sci. 2017, 55, 429–435. [Google Scholar] [CrossRef]
- Affam, A.C.; Chaudhuri, M. Degradation of pesticides chlorpyrifos, cypermethrin and chlorothalonil in aqueous solution by TiO2 photocatalysis. J. Environ. Manage. 2013, 130, 160–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Chen, F.; Ge, Y.; Wu, J.; Hu, X. Degradation behavior and products of malathion and chlorpyrifos spiked in apple juice by ultrasonic treatment. Ultrason. Sonochem. 2010, 17, 72–77. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Sequeira, R.; Alvarado-Hidalgo, F.; Robles-Chaves, D.; Sáenz-Arce, G.; Avendano-Soto, E.D.; Sánchez-Kopper, A.; Starbird-Perez, R. Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles. Polymers 2019, 11, 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091449
Zamora-Sequeira R, Alvarado-Hidalgo F, Robles-Chaves D, Sáenz-Arce G, Avendano-Soto ED, Sánchez-Kopper A, Starbird-Perez R. Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles. Polymers. 2019; 11(9):1449. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091449
Chicago/Turabian StyleZamora-Sequeira, Roy, Fernando Alvarado-Hidalgo, Diana Robles-Chaves, Giovanni Sáenz-Arce, Esteban D. Avendano-Soto, Andrés Sánchez-Kopper, and Ricardo Starbird-Perez. 2019. "Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles" Polymers 11, no. 9: 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091449