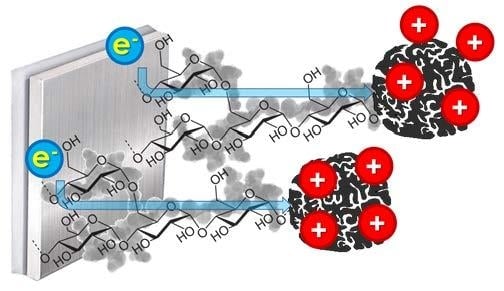
Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999; ISBN 978-1-4757-3060-9. [Google Scholar]
- Lu, M. Supercapacitors: Materials, Systems, and Applications; Béguin, F., Frąckowiak, E., Eds.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527328833. [Google Scholar]
- Helmholtz, H. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Annalen der Physik 1853, 165, 211–233. [Google Scholar] [CrossRef]
- Stern, O. Zur Theorie Der Elektrolytischen Doppelschicht. Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie 1924, 30, 508–516. [Google Scholar] [CrossRef]
- Koresh, J. Double Layer Capacitance and Charging Rate of Ultramicroporous Carbon Electrodes. J. Electrochem. Soc. 1977, 124, 1379. [Google Scholar] [CrossRef]
- Salitra, G.; Soffer, A.; Eliad, L.; Cohen, Y.; Aurbach, D. Carbon Electrodes for Double-Layer Capacitors I. Relations Between Ion and Pore Dimensions. J. Electrochem. Soc. 2000, 147, 2486. [Google Scholar] [CrossRef]
- Nagy, T.; Henderson, D.; Boda, D. Simulation of an Electrical Double Layer Model with a Low Dielectric Layer between the Electrode and the Electrolyte. J. Phys. Chem. B 2011, 115, 11409–11419. [Google Scholar] [CrossRef]
- Huang, J.; Sumpter, B.G.; Meunier, V. Theoretical Model for Nanoporous Carbon Supercapacitors. Angew. Chem. Int. Ed. 2008, 47, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Frackowiak, E.; Lota, G.; Machnikowski, J.; Vix-Guterl, C.; Béguin, F. Optimisation of supercapacitors using carbons with controlled nanotexture and nitrogen content. Electrochim. Acta 2006, 51, 2209–2214. [Google Scholar] [CrossRef]
- Stoeckli, F.; Centeno, T.A. On the determination of surface areas in activated carbons. Carbon 2005, 43, 1184–1190. [Google Scholar] [CrossRef] [Green Version]
- Hulicova, D.; Kodama, M.; Hatori, H. Electrochemical Performance of Nitrogen-Enriched Carbons in Aqueous and Non-Aqueous Supercapacitors. Chem. Mater. 2006, 18, 2318–2326. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Chmiola, J. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy Environ. Sci. 2012, 5, 9611. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Abbas, Q.; Babuchowska, P.; Frąckowiak, E.; Béguin, F. Sustainable AC/AC hybrid electrochemical capacitors in aqueous electrolyte approaching the performance of organic systems. J. Power Sources 2016, 326, 652–659. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Schütter, C.; Rojo, J.M.; Passerini, S.; Balducci, A.; Centeno, T.A. On the interaction of carbon electrodes and non conventional electrolytes in high-voltage electrochemical capacitors. J. Solid State Electrochem. 2018, 22, 717–725. [Google Scholar] [CrossRef]
- Aradilla, D.; Gao, F.; Lewes-Malandrakis, G.; Müller-Sebert, W.; Gentile, P.; Boniface, M.; Aldakov, D.; Iliev, B.; Schubert, T.J.S.; Nebel, C.E.; et al. Designing 3D Multihierarchical Heteronanostructures for High-Performance On-Chip Hybrid Supercapacitors: Poly(3,4-(ethylenedioxy)thiophene)-Coated Diamond/Silicon Nanowire Electrodes in an Aprotic Ionic Liquid. ACS Appl. Mater. Interfaces 2016, 8, 18069–18077. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Peng, C.; Chae, J.H.; Ng, K.C.; Chen, G.Z. Cell voltage versus electrode potential range in aqueous supercapacitors. Sci. Rep. 2015, 5, 9854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeżowski, P.; Fic, K.; Crosnier, O.; Brousse, T.; Béguin, F. Use of sacrificial lithium nickel oxide for loading graphitic anode in Li-ion capacitors. Electrochim. Acta 2016, 206, 440–445. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Menocal, S.; Amatucci, G. A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 2003, 115, 171–178. [Google Scholar] [CrossRef]
- Jeżowski, P.; Fic, K.; Crosnier, O.; Brousse, T.; Béguin, F. Lithium rhenium(VII) oxide as a novel material for graphite pre-lithiation in high performance lithium-ion capacitors. J. Mater. Chem. A 2016, 4, 12609–12615. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Isobe, Y.; Aoyagi, S. High-rate nano-crystalline Li4Ti5O12 attached on carbon nano-fibers for hybrid supercapacitors. J. Power Sources 2010, 195, 6250–6254. [Google Scholar] [CrossRef]
- Jeżowski, P.; Crosnier, O.; Deunf, E.; Poizot, P.; Béguin, F.; Brousse, T. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 2018, 17, 167–173. [Google Scholar] [CrossRef]
- Aida, T.; Murayama, I.; Yamada, K.; Morita, M. Improvement in Cycle Performance of a High-Voltage Hybrid Electrochemical Capacitor. Electrochem. Solid-State Lett. 2007, 10, A93. [Google Scholar] [CrossRef]
- Böckenfeld, N.; Jeong, S.S.; Winter, M.; Passerini, S.; Balducci, A. Natural, cheap and environmentally friendly binder for supercapacitors. J. Power Sources 2013, 221, 14–20. [Google Scholar] [CrossRef]
- Krause, A.; Balducci, A. High voltage electrochemical double layer capacitor containing mixtures of ionic liquids and organic carbonate as electrolytes. Electrochem. Commun. 2011, 13, 814–817. [Google Scholar] [CrossRef]
- Krause, A.; Kossyrev, P.; Oljaca, M.; Passerini, S.; Winter, M.; Balducci, A. Electrochemical double layer capacitor and lithium-ion capacitor based on carbon black. J. Power Sources 2011, 196, 8836–8842. [Google Scholar] [CrossRef]
- Kolodziej, A.; Fic, K.; Frackowiak, E. Towards sustainable power sources: Chitin-bound carbon electrodes for electrochemical capacitors. J. Mater. Chem. A 2015, 3, 22923–22930. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Northrop, P.W.C.; Crothers, A.C.; Jain, S.; Subramanian, V.R. Chitosan hydrogel-based electrode binder and electrolyte membrane for EDLCs: Experimental studies and model validation. J. Appl. Electrochem. 2012, 42, 935–943. [Google Scholar] [CrossRef]
- Varzi, A.; Raccichini, R.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Passerini, S. Probing the characteristics of casein as green binder for non-aqueous electrochemical double layer capacitors’ electrodes. J. Power Sources 2016, 326, 672–679. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. Proton-conducting polymer electrolytes and their applications in solid supercapacitors: A review. RSC Adv. 2014, 4, 33091–33113. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, B.K. Carbon nanotube–ZnO nanocomposite electrodes for supercapacitors. Solid State Ion. 2009, 180, 1525–1528. [Google Scholar] [CrossRef]
- Varzi, A.; Passerini, S. Enabling high areal capacitance in electrochemical double layer capacitors by means of the environmentally friendly starch binder. J. Power Sources 2015, 300, 216–222. [Google Scholar] [CrossRef]
- Portet, C.; Taberna, P.; Simon, P.; Laberty-Robert, C. Modification of Al current collector surface by sol–gel deposit for carbon–carbon supercapacitor applications. Electrochim. Acta 2004, 49, 905–912. [Google Scholar] [CrossRef]
- Taberna, P.L.; Portet, C.; Simon, P. Electrode surface treatment and electrochemical impedance spectroscopy study on carbon/carbon supercapacitors. Appl. Phys. A 2006, 82, 639–646. [Google Scholar] [CrossRef]
- Jeżowski, P.; Nowicki, M.; Grzeszkowiak, M.; Czajka, R.; Béguin, F. Chemical etching of stainless steel 301 for improving performance of electrochemical capacitors in aqueous electrolyte. J. Power Sources 2015, 279, 555–562. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Makowska, A.; Szwengiel, A.; Kubiak, P.; Tomaszewska-Gras, J. Characteristics and structure of starch isolated from triticale. Starch Stärke 2014, 66, 895–902. [Google Scholar] [CrossRef]
- Kennedy, H.M. Starch- and Dextrin-Based Adhesives. In Adhesives from Renewable Resources; ACS Publication: Washington, DC, USA, 1989; pp. 326–336. [Google Scholar]
- Imam, S.H.; Gordon, S.H.; Mao, L.; Chen, L. Environmentally friendly wood adhesive from a renewable plant polymer: Characteristics and optimization. Polym. Degrad. Stab. 2001, 73, 529–533. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Gu, Z.; Hong, Y.; Cheng, L. Preparation, characterization and properties of starch-based wood adhesive. Carbohydr. Polym. 2012, 88, 699–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh-Blicharz, J.; Lubiewski, Z.; Voelkel, E.; Lewandowicz, G. Evaluation of rheological properties of commercial native starches. Zywnosc Nauka Technologia Jakosc/Food Sci. Technol. Qual. 2011, 18, 53–65. [Google Scholar] [CrossRef]
- Makowska, A.; Kubiak, P.; Bialas, W.; Lewandowicz, G. Effect of urea, sodium nitrate and ethylene glycol addition on the rheological properties of corn starch pastes. Polimery 2015, 60, 343–350. [Google Scholar] [CrossRef]
- Kilbride, P.; Rull, M.V.; Townsend, A.; Wilson, H.; Morris, J. Shear-thickening fluids in biologically relevant agents. Biorheology 2019, 1–12. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Tong, J.; Zhang, X.; Peng, J.; Liu, X. Rheological Properties of Corn Starch Dispersions in Pregelatinized Starch Solution. In Proceedings of the 2018 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Hangzhou, China, 13–17 August 2018; pp. 146–150. [Google Scholar]
- Ai, Y.; Jane, J. Gelatinization and rheological properties of starch. Starch Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Baranowska, H.M.; Sikora, M.; Krystyjan, M.; Tomasik, P. Analysis of the formation of starch—Hydrocolloid binary gels and their structure based on the relaxation times of the water molecules. Polimery 2011, 56, 478–483. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold Plasma: An Alternative Technology for the Starch Modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Truong, V.-D.; Wang, L. Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohydr. Polym. 2003, 52, 327–333. [Google Scholar] [CrossRef]
- Richner, R.; Müller, S.; Bärtschi, M.; Kötz, R.; Wokaun, A. Physically and Chemically Bonded Carbonaceous Material for Double-Layer Capacitor Applications. J. New Mater. Electrochem. Syst. 2002, 5, 297–304. [Google Scholar]
- Maletin, Y.; Strelko, V.; Stryzhakova, N.; Zelinsky, S.; Rozhenko, A.B.; Gromadsky, D.; Volkov, V.; Tychina, S.; Gozhenko, O.; Drobny, D. Carbon Based Electrochemical Double Layer Capacitors of Low Internal Resistance. Energy Environ. Res. 2013, 3. [Google Scholar] [CrossRef]
- Maletin, Y.; Stryzhakova, N.; Zelinsky, S.; Chernukhin, S.; Tretyakov, D.; Tychina, S.; Drobny, D. Electrochemical Double Layer Capacitors and Hybrid Devices for Green Energy Applications. Green 2014, 4. [Google Scholar] [CrossRef]
- Yurii Maletin, N.S.; Sergii Zelinskyi, S.C.; Dmytro Tretyakov, H.M.; Natalia, D.; Dmytro, D. New Approach to Ultracapacitor Technology: What it Can Offer to Electrified Vehicles. J. Energy Power Eng. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Fuertes, A.B. Direct Synthesis of Highly Porous Interconnected Carbon Nanosheets and Their Application as High-Performance Supercapacitors. ACS Nano 2014, 8, 5069–5078. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Balducci, A. Theoretical and practical energy limitations of organic and ionic liquid-based electrolytes for high voltage electrochemical double layer capacitors. J. Power Sources 2014, 250, 343–351. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Quade, A.; Kickelbick, G.; Presser, V. Influence of Nitrogen-Doping for Carbide-Derived Carbons on the Supercapacitor Performance in an Organic Electrolyte and an Ionic Liquid. Batter. Supercaps 2018, 1, 135–148. [Google Scholar] [CrossRef]
- Krüner, B.; Lee, J.; Jäckel, N.; Tolosa, A.; Presser, V. Sub-micrometer Novolac-Derived Carbon Beads for High Performance Supercapacitors and Redox Electrolyte Energy Storage. ACS Appl. Mater. Interfaces 2016, 8, 9104–9115. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101648
Jeżowski P, Kowalczewski PŁ. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers. 2019; 11(10):1648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101648
Chicago/Turabian StyleJeżowski, Paweł, and Przemysław Łukasz Kowalczewski. 2019. "Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors" Polymers 11, no. 10: 1648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101648