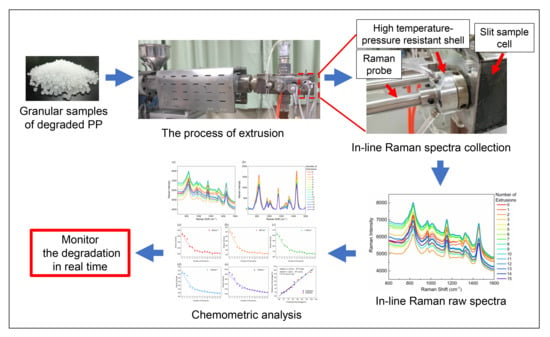
In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. In-Line Raman Spectroscopy Measurement
2.3. Off-Line Methods and Measurement
3. Results and Discussion
3.1. Analysis of In-Line Raman Spectra
3.2. Degradation Assessment of PP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Tocháček, J.; Jančář, J.; Kalfus, J.; Hermanová, S. Processing stability of polypropylene impact-copolymer during multiple extrusion-Effect of polymerization technology. Polym. Degrad. Stab. 2011, 96, 491–498. [Google Scholar] [CrossRef]
- Saw, L.T.; Uy Lan, D.N.; Rahim, N.A.A.; Mohd Kahar, A.W.; Viet, C.X. Processing degradation of polypropylene-ethylene copolymer-kaolin composites by a twin-screw extruder. Polym. Degrad. Stab. 2015, 111, 32–37. [Google Scholar] [CrossRef]
- Rajan, V.V.; Waeber, R.; Wieser, J. Online Monitoring of the Thermal Degradation of POM During Melt Extrusion. J. Appl. Polym. Sci. 2010, 115, 2394–2401. [Google Scholar] [CrossRef]
- da Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Ramírez-Vargas, E.; Navarro-Rodríguez, D.; Blanqueto-Menchaca, A.I.; Huerta-Martínez, B.M.; Palacios-Mezta, M. Degradation effects on the rheological and mechanical properties of multi-extruded blends of impact-modified polypropylene and poly(ethylene-co-vinyl acetate). Polym. Degrad. Stab. 2004, 86, 301–307. [Google Scholar] [CrossRef]
- Tayal, A.; Khan, S.A. Degradation of a Water-Soluble Polymer: Molecular Weight Changes and Chain Scission Characteristics. Macromolecules 2008, 33, 9488–9493. [Google Scholar] [CrossRef]
- Iedema, P.D.; Remerie, K.; van der Ham, M.; Biemond, E.; Tacx, J. Controlled peroxide-induced degradation of polypropylene in a twin-screw extruder: Change of molecular weight distribution under conditions controlled by micromixing. Chem. Eng. Sci. 2011, 66, 5474–5486. [Google Scholar] [CrossRef]
- Mylläri, V.; Ruoko, T.; Syrjälä, S. A comparison of rheology and FTIR in the study of polypropylene and polystyrene photodegradation. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Curcuruto, G.; Passaglia, E.; Gambarotti, C.; Dintcheva, N.T. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state-Part II: Thermal and thermo-oxidative degradation of polyamide 11/organo-clay nanocomposites. Polymer (Guildf.) 2015, 73, 102–110. [Google Scholar] [CrossRef]
- Barranco-García, R.; Cerrada, M.L.; Ressia, J.A.; Vallés, E.M.; García-Peñas, A.; Pérez, E.; Gómez-Elvira, J.M. Effect of mesoporous SBA-15 silica on the thermal stability of isotactic polypropylene based nanocomposites prepared by melt extrusion. Polym. Degrad. Stab. 2018, 154, 211–221. [Google Scholar] [CrossRef]
- Wang, Y.; Steinhoff, B.; Brinkmann, C.; Alig, I. In-line monitoring of the thermal degradation of poly(l-lactic acid) during melt extrusion by UV-vis spectroscopy. Polymer (Guildf.) 2008, 49, 1257–1265. [Google Scholar] [CrossRef]
- Montano-Herrera, L.; Pratt, S.; Arcos-Hernandez, M.V.; Halley, P.J.; Lant, P.A.; Werker, A.; Laycock, B. In-line monitoring of thermal degradation of PHA during melt-processing by Near-Infrared spectroscopy. N. Biotechnol. 2014, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hamester, L.S.; Muñoz, P.A.R.; Canevarolo, S.V. A new device for in-line colorimetric quantification of polypropylene degradation under multiple extrusions. Polym. Test. 2015, 41, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Saerens, L.; Dierickx, L.; Lenain, B.; Vervaet, C.; Remon, J.P.; De Beer, T. Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur. J. Pharm. Biopharm. 2011, 77, 158–163. [Google Scholar] [CrossRef]
- Netchacovitch, L.; Thiry, J.; De Bleye, C.; Dumont, E.; Cailletaud, J.; Sacré, P.Y.; Evrard, B.; Hubert, P.; Ziemons, E. Global approach for the validation of an in-line Raman spectroscopic method to determine the API content in real-time during a hot-melt extrusion process. Talanta 2017, 171, 45–52. [Google Scholar] [CrossRef]
- Kindermann, C.; Matthée, K.; Strohmeyer, J.; Sievert, F.; Breitkreutz, J. Tailor-made release triggering from hot-melt extruded complexes of basic polyelectrolyte and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2011, 79, 372–381. [Google Scholar] [CrossRef]
- Almeida, A.; Saerens, L.; De Beer, T.; Remon, J.P.; Vervaet, C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. Int. J. Pharm. 2012, 439, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Saerens, L.; Ghanam, D.; Raemdonck, C.; Francois, K.; Manz, J.; Krüger, R.; Krüger, S.; Vervaet, C.; Remon, J.P.; De Beer, T. In-line solid state prediction during pharmaceutical hot-melt extrusion in a 12 mm twin screw extruder using Raman spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Van Renterghem, J.; Kumar, A.; Vervaet, C.; Remon, J.P.; Nopens, I.; Vander Heyden, Y.; De Beer, T. Elucidation and visualization of solid-state transformation and mixing in a pharmaceutical mini hot melt extrusion process using in-line Raman spectroscopy. Int. J. Pharm. 2017, 517, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, M.J. Quantitative analysis using Raman spectrometry. Appl. Spectrosc. 2003, 57, 20A. [Google Scholar] [CrossRef] [PubMed]
- Frauendorfer, E.; Wolf, A.; Hergeth, W. Polymerization Online Monitoring. Chem. Eng. Technol. 2010, 33, 1767–1778. [Google Scholar] [CrossRef]
- Huang, X.; Lei, Y.; Wang, M.; Jin, G. In-line monitoring of component content of polypropylene/polystyrene blends during melt extrusion using Raman spectroscopy. J. Raman Spectrosc. 2018, 49, 513–519. [Google Scholar] [CrossRef]
- Wei, D.; Chen, S.; Liu, Q. Review of fluorescence suppression techniques in Raman spectroscopy. Appl. Spectrosc. Rev. 2015, 50, 387–406. [Google Scholar] [CrossRef]
- Hiejima, Y.; Kida, T.; Takeda, K.; Igarashi, T.; Nitta, K.H. Microscopic structural changes during photodegradation of low-density polyethylene detected by Raman spectroscopy. Polym. Degrad. Stab. 2018, 150, 67–72. [Google Scholar] [CrossRef]
- González-González, V.A.; Neira-Velázquez, G.; Angulo-Sánchez, J.L. Polypropylene chain scissions and molecular weight changes in multiple extrusion *. Polym. Degrad. Stab. 1998, 60, 33–42. [Google Scholar] [CrossRef]
- Nasir, A.; Yasin, T.; Islam, A. Thermo-oxidative degradation behavior of recycled polypropylene. J. Appl. Polym. Sci. 2011, 119, 3315–3320. [Google Scholar] [CrossRef]
- Khafagy, R.M. In situ FT-Raman spectroscopic study of the conformational changes occurring in isotactic polypropylene during its melting and crystallization processes. J. Polym. Sci. Part. B Polym. Phys. 2006, 44, 2173–2182. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly (propylene) Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly (propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Chan, J. The thermal degradation kinetics of polypropylene: Part I. Molecular weight distribution. Polym. Degrad. Stab. 1997, 57, 113–125. [Google Scholar] [CrossRef]
- Sebstiao, V. Canevarolo, Chain scission distribution function for polypropylene degradation during multiple extrusions. Polym. Degrad. Stab. 2000, 709, 71–76. [Google Scholar]








| Raman Characteristic Band (cm−1) | Vibration Modes |
|---|---|
| 832 | C–C stretching, CH3 rocking |
| 967 | C–C stretching, CH3 rocking |
| 1150 | C–C stretching, CH bending |
| 1324 | CH stretching, CH2 wagging, CH3 bending |
| 1454 | CH2 bending, CH3 asymmetric bending |
| Raman main characteristic band of PP/cm−1 | 832 | 967 | 1150 | 1324 | 1454 |
| The linear correlation coefficient (R) | 0.936 | 0.912 | 0.955 | 0.926 | 0.958 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy. Polymers 2019, 11, 1698. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101698
Guo X, Lin Z, Wang Y, He Z, Wang M, Jin G. In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy. Polymers. 2019; 11(10):1698. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101698
Chicago/Turabian StyleGuo, Xuemei, Zenan Lin, Yingjun Wang, Zhangping He, Mengmeng Wang, and Gang Jin. 2019. "In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy" Polymers 11, no. 10: 1698. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101698