Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA Based Formulations
2.3. Characterization of PVA Based Formulations
2.4. GA and QC Release Studies in Food Simulant (Specific Migration)
2.5. Antioxidant Activity of Films and Food Simulant Solutions
2.5.1. Antioxidant Activity of Films
2.5.2. Antioxidant Activity of Food Simulant Solutions
2.6. Statistical Analysis
3. Results and Discussion
3.1. GA and QC Characterization
3.2. Characterization of PVA Based Formulations
3.2.1. Morphological Properties
3.2.2. Thermal Analysis
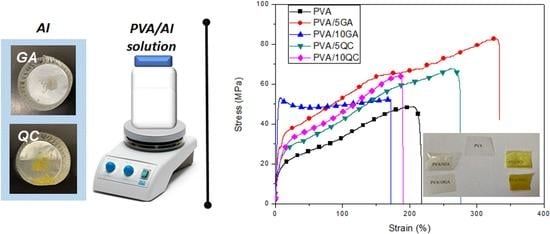
3.2.3. Mechanical Response
3.2.4. Overall Migration, Antioxidant Activity of PVA Formulations and Simulant Solutions at Different Times
3.2.5. Release Tests in Food Simulant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, T.M.; Romani, A.; Bernini, R.; Sweeney, T. Anti-Inflammatory Effects of Pomegranate Peel Extracts on In Vitro Human Intestinal Caco-2 Cells and Ex Vivo Porcine Colonic Tissue Explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Pannucci, E.; Caracciolo, R.; Romani, A.; Cacciola, F.; Dugo, P.; Bernini, R.; Varvaro, L.; Santi, L. An hydroxytyrosol enriched extract from olive mill wastewaters exerts antioxidant activity and antimicrobial activity on Pseudomonas savastanoi pv. savastanoi and Agrobacterium tumefaciens. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable Tannins from Sweet Chestnut Fractions Obtained by a Sustainable and Eco-friendly Industrial Process. Nat. Prod. Commun. 2016, 11, 1934578X1601100323. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Shao, L.; Dong, D.; Wang, F.; Zhang, Y.; Wang, Y. Bio-inspired natural polyphenol cross-linking poly (vinyl alcohol) films with strong integrated strength and toughness. RSC Adv. 2016, 6, 69966–69972. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In Plant Based “Green Chemistry 2.0”. Green Chemistry and Sustainable Technology; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Fortunati, E.; Luzi, F.; Fanali, C.; Dugo, L.; Belluomo, M.G.; Torre, L.; Kenny, J.M.; Santi, L.; Bernini, R. Hydroxytyrosol as Active Ingredient in Poly (vinyl alcohol) Films for Food Packaging Applications. J. Renew. Mater. 2016, 5, 81–95. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef]
- Xu, X.-J.; Huang, S.-M.; Zhang, L.-H. Biodegradability, antibacterial properties, and ultraviolet protection of polyvinyl alcohol-natural polyphenol blends. Polym. Compos. 2009, 30, 1611–1617. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Ottombrino, A.; Fratianni, F.; Nazzaro, F. Antibacterial properties of polyvinyl alcohol-rosemary polyphenol blends. In Microbes in Applied Research; WORLD SCIENTIFIC: Singapore, 2012; pp. 209–212. [Google Scholar]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Wu, J.-G.; Wang, P.-J.; Chen, S.C. Antioxidant and Antimicrobial Effectiveness of Catechin-Impregnated PVA–Starch Film on Red Meat. J. Food Qual. 2010, 33, 780–801. [Google Scholar] [CrossRef]
- de Carvalho, S.M.; Noronha, C.M.; da Rosa, C.G.; Sganzerla, W.G.; Bellettini, I.C.; Nunes, M.R.; Bertoldi, F.C.; Manique Barreto, P.L. PVA antioxidant nanocomposite films functionalized with alpha-tocopherol loaded solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123793. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Chen, Z.; Liu, S.; Li, J.; Li, S. Natural Quercetin AIEgen Composite Film with Antibacterial and Antioxidant Properties for in Situ Sensing of Al3+ Residues in Food, Detecting Food Spoilage, and Extending Food Storage Times. ACS Appl. Bio Mater. 2018, 1, 636–642. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Giovanale, G.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Cellulose nanocrystals from Actinidia deliciosa pruning residues combined with carvacrol in PVA_CH films with antioxidant/antimicrobial properties for packaging applications. Int. J. Biol. Macromol. 2017, 104, 43–55. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- da Costa, E.M.; Filho, J.M.B.; do Nascimento, T.G.; Macêdo, R.O. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 2002, 392–393, 79–84. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Carini, J.P.; Honorato, S.B.; Ayala, A.P.; Moreira, J.C.F.; Bassani, V.L. Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochim. Acta 2012, 539, 109–114. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E. Effect of UV-A irradiation and temperature on the antioxidant activity of quercetin studied using ABTS, DPPH and electrochemistry methods. Int. J. Electrochem. Sci. 2015, 10, 5276–5290. [Google Scholar]
- Bruno, F.F.; Trotta, A.; Fossey, S.; Nagarajan, S.; Nagarajan, R.; Samuelson, L.A.; Kumar, J. Enzymatic Synthesis and Characterization of PolyQuercetin. J. Macromol. Sci. Part A 2010, 47, 1191–1196. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Woźnicka, E.; Pieniążek, E.; Zapała, L.; Byczyński, Ł.; Trojnar, I.; Kopacz, M. New sulfonic derivatives of quercetin as complexing reagents: Synthesis, spectral, and thermal characterization. J. Therm. Anal. Calorim. 2015, 120, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.B.; Silva, A.M.D.; Barroca, M.J.; Marques, M.P.M.; Braga, S.S. Physicochemical properties, antioxidant action and practical application in fresh cheese of the solid inclusion compound γ-cyclodextrin·quercetin, in comparison with β-cyclodextrin·quercetin. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Dixit, S. Quercetin attenuates the development of 7, 12-dimethyl benz (a) anthracene (DMBA) and croton oil-induced skin cancer in mice. J. Biomed. Res. 2015, 29, 139. [Google Scholar]
- Sarria-Villa, R.A.; Gallo-Corredor, J.A.; Páez, M.I. Isolation of catechin and gallic acid from Colombian bark of Pinus patula. Chem. Sci. J. 2017, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Omrani, Z.; Tehrani, A.D. New cyclodextrin-based supramolecular nanocapsule for codelivery of curcumin and gallic acid. Polym. Bull. 2019, 1–17. [Google Scholar] [CrossRef]
- Naz, S.; Khaskheli, A.R.; Aljabour, A.; Kara, H.; Talpur, F.N.; Sherazi, S.T.H.; Khaskheli, A.A.; Jawaid, S. Synthesis of highly stable cobalt nanomaterial using gallic acid and its application in catalysis. Adv. Chem. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, C.; Ma, H.; Yuan, E.; Li, Q. Characterization and DPPH radical scavenging activity of gallic acid-lecithin complex. Trop. J. Pharm. Res. 2014, 13, 1333–1338. [Google Scholar] [CrossRef] [Green Version]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Gohla, S.; Li, L.; Müller, R.H. Long-term stability of quercetin nanocrystals prepared by different methods. J. Pharm. Pharmacol. 2012, 64, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, Y.-H.; Yu, D.-G.; Williams, G.R.; Huang, S.-M.; Tao, W.; Sun, J.-Y. Electrospun acid–base pair solid dispersions of quercetin. RSC Adv. 2014, 4, 58265–58271. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin Nanosuspensions Produced by High-Pressure Homogenization. J. Agric. Food Chem. 2014, 62, 1852–1859. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef]
- Daneshfar, A.; Ghaziaskar, H.S.; Homayoun, N. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. J. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- Oliveira, R.N.; McGuinness, G.B.; Rouze, R.; Quilty, B.; Cahill, P.; Soares, G.D.A.; Thiré, R.M.S.M. PVA hydrogels loaded with a Brazilian propolis for burn wound healing applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Peng, M.; Xiao, G.; Tang, X.; Zhou, Y. Hydrogen-Bonding Assembly of Rigid-Rod Poly(p-sulfophenylene terephthalamide) and Flexible-Chain Poly(vinyl alcohol) for Transparent, Strong, and Tough Molecular Composites. Macromolecules 2014, 47, 8411–8419. [Google Scholar] [CrossRef]
- Zhu, B.; Li, J.; He, Y.; Yoshie, N.; Inoue, Y. Hydrogen-Bonding Interaction and Crystalline Morphology in the Binary Blends of Poly(ε-caprolactone) and Polyphenol Catechin. Macromol. Biosci. 2003, 3, 684–693. [Google Scholar] [CrossRef]
- Thomas, P.S.; Guerbois, J.P.; Russell, G.F.; Briscoe, B.J. FTIR study of the thermal degradation of poly (vinyl alcohol). J. Therm. Anal. Calorim. 2001, 64, 501–508. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhand, C.; Harini, S.; Venkatesh, M.; Dwivedi, N.; Ng, A.; Liu, S.; Verma, N.K.; Ramakrishna, S.; Beuerman, R.W.; Loh, X.J.; et al. Multifunctional Polyphenols- and Catecholamines-Based Self-Defensive Films for Health Care Applications. ACS Appl. Mater. Interfaces 2016, 8, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.-S.; Vallons, K.J.R.; Arendt, E.K. Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J. Agric. Food Chem. 2012, 60, 6157–6163. [Google Scholar] [CrossRef]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total Phenolics and Antioxidant Activities of Fenugreek, Green Tea, Black Tea, Grape Seed, Ginger, Rosemary, Gotu Kola, and Ginkgo Extracts, Vitamin E, and tert-Butylhydroquinone. J. Agric. Food Chem. 2004, 52, 5183–5186. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Yoon, S.-D.; Chough, S.-H.; Park, H.-R. Effects of additives with different functional groups on the physical properties of starch/PVA blend film. J. Appl. Polym. Sci. 2006, 100, 3733–3740. [Google Scholar] [CrossRef]
- Schmidt, B.; Katiyar, V.; Plackett, D.; Larsen, E.H.; Gerds, N.; Koch, C.B.; Petersen, J.H. Migration of nanosized layered double hydroxide platelets from polylactide nanocomposite films. Food Addit. Contam. Part A 2011, 28, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Effect of hydroxytyrosol methyl carbonate on the thermal, migration and antioxidant properties of PVA-based films for active food packaging. Polym. Int. 2016, 65, 872–882. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Ruseckaite, R.A.; Vázquez, A. Kinetic analysis of thermal degradation in poly(ethylene–vinyl alcohol) copolymers. J. Appl. Polym. Sci. 2003, 90, 3157–3163. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Silva, H.D.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Development and Characterization of an Active Chitosan-Based Film Containing Quercetin. Food Bioprocess Technol. 2015, 8, 2183–2191. [Google Scholar] [CrossRef] [Green Version]
- Braga, L.R.; Pérez, L.M.; Soazo, M.D.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- European, C. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Zuo, B.; Hu, Y.; Lu, X.; Zhang, S.; Fan, H.; Wang, X. Surface Properties of Poly(vinyl alcohol) Films Dominated by Spontaneous Adsorption of Ethanol and Governed by Hydrogen Bonding. J. Phys. Chem. C 2013, 117, 3396–3406. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural Biomaterial-Based Edible and pH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef] [PubMed]









| Cooling | ||||
|---|---|---|---|---|
| Formulations | Tg (°C) | ΔHc (J g−1) | Tc (°C) | Xc (%) |
| PVA | 73.1 ± 0.1 b | 48.5 ± 2.1 a,b | 188.9 ± 0.2 a | 30.0 ± 1.3 a,b |
| PVA/5GA | 71.9 ± 0.5 a,b | 54.0 ± 2.3 c | 201.1 ± 0.5 c | 33.4 ± 1.3 c |
| PVA/10GA | 71.6 ± 0.8 a,b | 48.09 ± 0.3 a,b | 198.0 ± 2.0 b | 29.8 ± 0.2 a,b |
| PVA/5QC | 71.5 ± 0.3 a | 52.0 ± 0.3 b,c | 200.9 ± 0.2 c | 32.2 ± 0.2 b,c |
| PVA/10QC | 71.1 ± 0.2 a | 45.3 ± 2.1 a | 191.5 ± 0.9 a | 28.1 ± 1.3 a |
| Second Heating | ||||
| Tg (°C) | ΔHm (J g−1) | Tm (°C) | Xm (%) | |
| PVA | 76.6 ± 0.9 a | 38.0 ± 3.9 a | 217.3 ± 0.1 a | 23.5 ± 2.4 a |
| PVA/5GA | 82.6 ± 0.1 b | 56.7 ± 2.1 b | 227.5 ± 0.2 c | 35.1 ± 1.3 b |
| PVA/10GA | 83.8 ± 1.0 b,c | 53.9 ± 2.7 b | 223.7 ± 0.6 b | 33.3 ± 1.7 b |
| PVA/5QC | 84.9 ± 0.3 c | 50.9 ± 1.8 b | 227.9 ± 0.6 c | 31.5 ± 1.2 b |
| PVA/10QC | 84.5 ± 0.1 b,c | 58.0 ± 3.6 b | 224.6 ± 0.5 b | 32.281 ± 2.0 b |
| Formulations | Thickness (Mean Value, μm) | σb (MPa) | εb (%) | E (MPa) |
|---|---|---|---|---|
| PVA | 45 ± 5 b | 67 ± 12 a,b | 195 ± 45 a,b | 300 ± 80 a |
| PVA/5GA | 40 ± 5 a | 75 ± 10 b | 280 ± 70 c | 750 ± 90 b |
| PVA/10GA | 50 ± 8 c | 55 ± 10 a | 190 ± 80 a,b | 2000 ± 325 c |
| PVA/5QC | 40 ± 7 a | 74 ± 10 b | 255 ± 35 b,c | 305 ± 50 a |
| PVA/10QC | 50 ± 7 c | 60 ± 10 a | 170 ± 10 a | 520 ± 110 a,b |
| Formulations | Overall Migration in Ethanol 50% (v/v) (mg kg−1) |
|---|---|
| PVA | 4.1 ± 0.3 a |
| PVA/5GA | 6.8 ± 0.1 b |
| PVA/10GA | 9.9 ± 0.5 c |
| PVA/5QC | 6.6 ± 0.4 b |
| PVA/10QC | 9.5 ± 0.4 c |
| Formulations | DPPH Scavenging Activity, RSA (%) | |||||
|---|---|---|---|---|---|---|
| Time 0 Day | Time 1 Day | Time 3 Days | Time 7 Days | Time 10 Days | Time 21 Days | |
| PVA/5GA | 90.7 ± 0.1 a | 94.3 ± 0.3 c | 93.8 ± 0.3 c | 95.0 ± 0.3 d | 94.1 ± 0.3 c | 92.7 ± 0.3 b |
| PVA/10GA | 95.3 ± 0.2 b | 95.6 ± 0.2 b | 95.0 ± 0.3 a,b | 95.1 ± 0.1 b | 95.6 ± 0.3 b | 94.5 ± 0.2 a |
| PVA/5QC | 89.0 ± 0.1 a | 95.3 ± 0.2 c | 96.6 ± 0.3 e | 96.3 ± 0.2 d,e | 95.7 ± 0.2 c,d | 94.3 ± 0.5 b |
| PVA/10QC | 91.3 ± 0.2 a | 95.5 ± 0.3 c | 95.9 ± 02 c | 95.8 ± 0.1 c | 95.4 ± 0.2c | 94.5 ± 0.3 b |
| Formulations | L * | a * | b * | ∆E * | Gloss (°) |
|---|---|---|---|---|---|
| White Control | 99.47 ± 0.00 | −0.08 ± 0.01 | −0.08 ± 0.01 | - | 121 ± 0 |
| PVA | |||||
| Time 0 | 98.98 ± 0.09 c | −0.09 ± 0.00 b | 0.19 ± 0.02 a | 0.56 ± 0.09 a | 252 ± 3 b |
| Time 7 days | 98.52 ± 0.01 b | −0.17 ± 0.00 a | 0.46 ± 0.03 b | 1.09 ± 0.00 b | 219 ± 3 a |
| Time 21 days | 98.02 ± 0.08 a | −0.17 ± 0.01 a | 0.59 ± 0.02 c | 1.60 ± 0.06 b | 219 ± 6 a |
| PVA/5GA | |||||
| Time 0 | 96.77 ± 0.02 c | −0.61 ± 0.00 c | 5.66 ± 0.02 a | 6.36 ± 0.01 a | 234 ± 2 b |
| Time 7 days | 91.64 ± 0.17 b | −1.21 ± 0.08 b | 12.76 ± 0.15 b | 15.08 ± 0.21 b | 211 ± 9 b |
| Time 21 days | 85.77 ± 0.03 a | −3.64 ± 0.08 a | 17.21 ± 0.18 c | 22.34 ± 0.17 c | 165 ± 7 a |
| PVA/10GA | |||||
| Time 0 | 96.98 ± 0.04 c | −0.41 ± 0.05 a | 3.00 ± 0.02 a | 3.97 ± 0.05 a | 164 ± 0 b |
| Time 7 day | 95.23 ± 0.01 b | −0.64 ± 0.09 a | 6.24 ± 0.06 b | 7.63 ± 0.05 b | 138 ± 1 a |
| Time 21 days | 92.77 ± 0.62 a | −0.65 ± 0.09 a | 12.70 ± 0.62 c | 14.44 ± 0.82 c | 156 ± 10 b |
| PVA/5QC | |||||
| Time 0 | 90.84 ± 0.01 c | −9.10 ± 0.13 a | 62.73 ± 0.02 b | 64.03 ± 0.01 b | 52 ± 4 b |
| Time 7 days | 83.18 ± 0.01 b | 3.23 ± 0.02 b | 63.32 ± 0.04 c | 65.54 ± 0.04 c | 93 ± 2 c |
| Time 21 days | 76.91 ± 0.06 a | 14.95 ± 0.03 c | 50.67 ± 0.23 a | 57.23 ± 0.24 a | 29 ± 1 a |
| PVA/10QC | |||||
| Time 0 | 82.48 ± 0.43 b | −0.29 ± 0.08 a | 69.39 ± 0.01 c | 71.51 ± 0.10 b | 75 ± 3 a |
| Time 7 days | 87.04 ± 0.32 c | 1.47 ± 0.05 b | 50.58 ± 0.92 a | 52.19 ± 0.97 a | 101 ± 1 c |
| Time 21 days | 71.87 ± 0.42 a | 20.04 ± 0.39 c | 64.30 ± 0.82 b | 72.88 ± 0.99 b | 95 ± 1 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121999
Luzi F, Pannucci E, Santi L, Kenny JM, Torre L, Bernini R, Puglia D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers. 2019; 11(12):1999. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121999
Chicago/Turabian StyleLuzi, Francesca, Elisa Pannucci, Luca Santi, José Maria Kenny, Luigi Torre, Roberta Bernini, and Debora Puglia. 2019. "Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging" Polymers 11, no. 12: 1999. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121999