Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Electrospinning in the Production of Nanofibrous Scaffolds
| Composition | Natural (Nt) | Synthetic (St) | Blended |
| Collagen | Poly(vinyl alcohol) | Nt + St | |
| Chitosan | Poly(ε-caprolactone) | Nt + St + Coating | |
| Silk | Poly(lactic acid) | ||
| Alginate | Poly(lactic-co-glycolic acid) | ||
| Morphology | Solid | Porous | Core-Shell |
| Assembly | Random | Aligned | Layered |
Yarn | Hollow | Fiber Bundle |
3. Poly(Vinyl Alcohol)

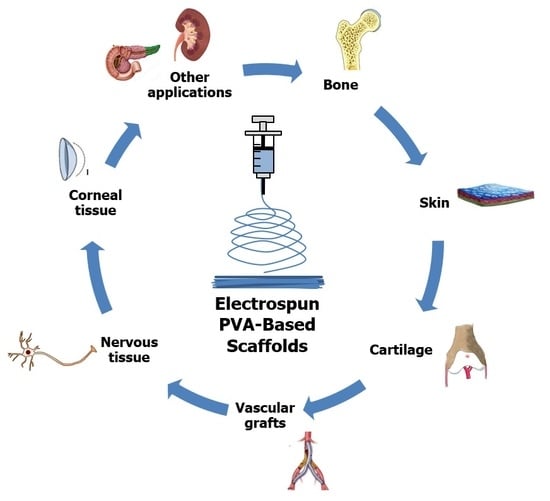
4. Tissue Engineering Applications of PVA-Based Nanofibrous Scaffolds
4.1. Bone
4.2. Skin
4.3. Cartilage
4.4. Vascular Grafts
4.5. Nervous Tissue
4.6. Corneal Tissue
4.7. Other Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tudu, M. Preparation of Composite Nanofibres for Bone Tissue Regeneration. Ph.D. Thesis, National Institute of Technology Rourkela, Rourkela, India, 2012. [Google Scholar]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Rafienia, M.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kolbuk, D.; Pahlevanneshan, Z.; Bonakdar, S.H. Development of electrospun poly (vinyl alcohol)-based bionanocomposite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 1111–1120. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225–1241. [Google Scholar]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Felgueiras, H.; Tavares, T.; Amorim, M. Biodegradable, Spun Nanocomposite Polymeric Fibrous Dressings Loaded with Bioactive Biomolecules for An Effective Wound Healing: A Review; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bangkok, Thailand, 2019. [Google Scholar]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2016, 61, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrabi, F.; Shamspur, T.; Mostafavi, A.; Saljooqi, A.; Fathirad, F. Synthesis of cellulose acetate nanofibers and its application in the release of some drugs. Nanomed. Res. J. 2017, 2, 199–207. [Google Scholar]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abazari, M.F.; Soleimanifar, F.; Aleagha, M.N.; Torabinejad, S.; Nasiri, N.; Khamisipour, G.; Mahabadi, J.A.; Mahboudi, H.; Enderami, S.E.; Saburi, E.; et al. PCL/PVA nanofibrous scaffold improve insulin-producing cells generation from human induced pluripotent stem cells. Gene 2018, 671, 50–57. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Golafshan, N.; Kharaziha, M.; Fathi, M. Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 2017, 111, 752–763. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Coelho, D.; Sampaio, A.; Silva, C.J.; Felgueiras, H.P.; Amorim, M.T.P.; Zille, A. Antibacterial electrospun poly (vinyl alcohol)/enzymatic synthesized poly (catechol) nanofibrous midlayer membrane for ultrafiltration. ACS Appl. Mater. Interfaces 2017, 9, 33107–33118. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lipner, J.; Moran, C.H.; Feng, L.; Li, X.; Thomopoulos, S.; Xia, Y. Generation of Electrospun Nanofibers with Controllable Degrees of Crimping Through a Simple, Plasticizer-Based Treatment. Adv. Mater. 2015, 27, 2583–2588. [Google Scholar] [CrossRef]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of corneal tissue through an aligned PVA/collagen composite nanofibrous electrospun scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosunmu, O.; Chase, G.G.; Kataphinan, W.; Reneker, D. Electrospinning of polymer nanofibres from multiple jets on a porous tubular surface. Nanotechnology 2006, 17, 1123. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Amorim, M. Electrospun Polymeric Dressings Functionalized with Antimicrobial Peptides and Collagen Type I for Enhanced Wound Healing; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Corfu, Greece, 2017. [Google Scholar]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Uslu, İ.; Tunç, T.; Öztürk, M.; Aytimur, A. Preparation and characterization of neodymia doped PVA/Zr-Ce oxide nanocrystalline composites via electrospinning technique. Mater. Manuf. Process. 2011, 26, 1346–1351. [Google Scholar] [CrossRef]
- Thompson, C.; Chase, G.G.; Yarin, A.; Reneker, D. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Wu, J.-W.; Wong, S.-C.; Qu, J.-P.; Srivatsan, T. The technique of electrospinning for manufacturing core-shell nanofibers. Mater. Manuf. Process. 2018, 33, 202–219. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar-Mohammadi, M.; Kargozar, S.; Bahrami, S.H.; Joghataei, M. Fabrication of curcumin-loaded gum tragacanth/poly (vinyl alcohol) nanofibers with optimized electrospinning parameters. J. Ind. Text. 2017, 46, 1170–1192. [Google Scholar] [CrossRef]
- Teixeira, M.; Amorim, M.; Felgueiras, H. PVA/CA Based Electrospun Nanofibers: Influence of Processing Parameters in the Fiber Diameter; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bangkok, Thailand, 2019. [Google Scholar]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; Supriyanto, E.; Razak, S.I.A. An insight on electrospun-nanofibers-inspired modern drug delivery system in the treatment of deadly cancers. Rsc Adv. 2015, 5, 57984–58004. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Gholipour-Kanani, A.; Bahrami, S.H.; Joghataie, M.T.; Samadikuchaksaraei, A.; Ahmadi-Taftie, H.; Rabbani, S.; Kororian, A.; Erfani, E. Tissue engineered poly(caprolactone)-chitosan-poly(vinyl alcohol) nanofibrous scaffolds for burn and cutting wound healing. IET Nanobiotechnol. 2014, 8, 123–131. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Shim, E.; Su, J.; Noro, J.; Teixeira, M.A.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Conductive bacterial cellulose by in situ laccase polymerization of aniline. PLoS ONE 2019, 14, e0214546. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials 2019, 9, 1064. [Google Scholar] [CrossRef] [Green Version]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M. Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci. Iran 2012, 19, 2029–2034. [Google Scholar] [CrossRef] [Green Version]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. 2016, 12, 2181–2200. [Google Scholar] [CrossRef] [PubMed]
- Varesano, A.; Carletto, R.A.; Mazzuchetti, G. Experimental investigations on the multi-jet electrospinning process. J. Mater. Process. Technol. 2009, 209, 5178–5185. [Google Scholar] [CrossRef]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. 11-Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 325–347. [Google Scholar]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Cavaliere, S.; Subianto, S.; Savych, I.; Jones, D.J.; Rozière, J. Electrospinning: Designed architectures for energy conversion and storage devices. Energ. Environ. Sci. 2011, 4, 4761–4785. [Google Scholar] [CrossRef] [Green Version]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. Rsc Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef] [Green Version]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef]
- Roman, M.P.; Thoppey, N.M.; Gorga, R.E.; Bochinski, J.R.; Clarke, L.I. Maximizing Spontaneous Jet Density and Nanofiber Quality in Unconfined Electrospinning: The Role of Interjet Interactions. Macromolecules 2013, 46, 7352–7362. [Google Scholar] [CrossRef]
- Jiang, G.; Qin, X. An improved free surface electrospinning for high throughput manufacturing of core–shell nanofibers. Mater. Lett. 2014, 128, 259–262. [Google Scholar] [CrossRef]
- Lin, T. Needleless electrospinning: A practical way to mass production of nanofibers. J. Text. Sci. Eng. 2012, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, L.; Sun, X.-F.; Liu, J.; Fan, J.; Huang, D.-W. Multi-jet electrospinning via auxiliary electrode. Mater. Lett. 2015, 141, 153–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Qi, Y.; Zhou, D.; Xie, Z.; Jing, X.; Chen, X.; Huang, Y. Time-programmed DCA and oxaliplatin release by multilayered nanofiber mats in prevention of local cancer recurrence following surgery. J. Control. Release 2016, 235, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, L.; Cui, X. Fabrication of Aligned Side-by-Side TiO2/SnO2 Nanofibers via Dual-Opposite-Spinneret Electrospinning. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, D.D.; Guo, P.; Leckie, J.O. An Efficient Bicomponent TiO2/SnO2 Nanofiber Photocatalyst Fabricated by Electrospinning with a Side-by-Side Dual Spinneret Method. Nano Lett. 2007, 7, 1081–1085. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion electrospinning of polycaprolactone: Influence of surfactant type towards the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, L.; Dempsey, E.M.; Song, W.; Qi, R.; Li, W.; Huang, Y.; Jing, X.; Zhou, D.; Ding, J. Recent progress in polymer-based platinum drug delivery systems. Prog. Polym. Sci. 2018, 87, 70–106. [Google Scholar] [CrossRef]
- Sy, J.C.; Klemm, A.S.; Shastri, V.P. Emulsion as a Means of Controlling Electrospinning of Polymers. Adv. Mater. 2009, 21, 1814–1819. [Google Scholar] [CrossRef]
- Stoddard, R.J.; Steger, A.L.; Blakney, A.K.; Woodrow, K.A. In pursuit of functional electrospun materials for clinical applications in humans. Ther. Deliv. 2016, 7, 387–409. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Y.; Huang, X.; Yue, T. Controlled release of protein from core–shell nanofibers prepared by emulsion electrospinning based on green chemical. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bilhim, T.; Pisco, J.M.; Duarte, M.; Oliveira, A.G. Polyvinyl Alcohol Particle Size for Uterine Artery Embolization: A Prospective Randomized Study of Initial Use of 350–500 μm Particles versus Initial Use of 500–700μm Particles. J. Vasc. Interv. Radiol. 2011, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.L.; Nguyen, V.H.; Haldorai, Y.; Shim, J.-J. Polymerization of vinyl pivalate in supercritical carbon dioxide and the saponification for the preparation of syndiotacticity-rich poly(vinyl alcohol). Korean J. Chem. Eng. 2013, 30, 1153–1161. [Google Scholar] [CrossRef]
- Horikoshi, T.; Ogawa, A.; Saito, T.; Hoshiro, H. Properties of Polyvinylalcohol fiber as reinforcing materials for cementitious composites. In International RILEM Workshop on High Performance Fiber Reinforced Cementitious Composites in Structural Applications; Fischer, G., Li, V.C., Eds.; RILEM Publications SARL: Bagneux, France, 2006. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Chaouat, M.; Le Visage, C.; Baille, W.E.; Escoubet, B.; Chaubet, F.; Mateescu, M.A.; Letourneur, D. A Novel Cross-linked Poly(vinyl alcohol) (PVA) for Vascular Grafts. Adv. Funct. Mater. 2008, 18, 2855–2861. [Google Scholar] [CrossRef]
- Thong, C.C.; Teo, D.C.L.; Ng, C.K. Application of polyvinyl alcohol (PVA) in cement-based composite materials: A review of its engineering properties and microstructure behavior. Constr. Build. Mater. 2016, 107, 172–180. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly (vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Akhtar, M.; Kadhum, A.; Mohamad, A.; Al-Amiery, A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Cebe, P. Self-nucleation and crystallization of polyvinyl alcohol. J. Therm. Anal. Calorim. 2017, 127, 885–894. [Google Scholar] [CrossRef]
- Briscoe, B.; Luckham, P.; Zhu, S. The effects of hydrogen bonding upon the viscosity of aqueous poly(vinyl alcohol) solutions. Polymer 2000, 41, 3851–3860. [Google Scholar] [CrossRef]
- Kasai, D.; Chougale, R.; Masti, S.; Chalannavar, R.; Malabadi, R.B.; Gani, R. Influence of Syzygium cumini leaves extract on morphological, thermal, mechanical, and antimicrobial properties of PVA and PVA/chitosan blend films. J. Appl. Polym. Sci. 2018, 135, 46188. [Google Scholar] [CrossRef]
- Sivakumar, M. Effect of Polymer modification on mechanical and structural properties of concrete—An experimental investigation. Int. J. Civ. Struct. Eng. 2011, 1, 732–740. [Google Scholar]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Marten, F.L. Vinyl Alcohol Polymers. In Encyclopedia of Polymer Science and Technology; Wiley Online Library: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Roy, S.; Kuddannaya, S.; Das, T.; Lee, H.Y.; Lim, J.; Hu, X.M.; Chee Yoon, Y.; Kim, J. A novel approach for fabricating highly tunable and fluffy bioinspired 3D poly(vinyl alcohol) (PVA) fiber scaffolds. Nanoscale 2017, 9, 7081–7093. [Google Scholar] [CrossRef]
- Xia, L.L.; Li, C.L.; Wang, Y. In-situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Sonker, A.K.; Verma, V. Influence of crosslinking methods toward poly(vinyl alcohol) properties: Microwave irradiation and conventional heating. J. Appl. Polym. Sci. 2018, 135, 46125. [Google Scholar] [CrossRef]
- Vashisth, P.; Pruthi, V. Synthesis and characterization of crosslinked gellan/PVA nanofibers for tissue engineering application. Mater. Sci. Eng. C 2016, 67, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J. Polym. Environ. 2018, 26, 1782–1794. [Google Scholar] [CrossRef]
- Langer, R. Biopolymers in Controlled Release Systems. In Polymeric Biomaterials; Piskin, E., Hoffman, A.S., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1986; pp. 161–169. [Google Scholar]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In Situ Cross-Linking of Electrospun Poly(vinyl alcohol) Nanofibers. Macromolecules 2010, 43, 630–637. [Google Scholar] [CrossRef]
- Chae, T.; Ko, F. 19-Electrospun nanofibrous tissue scaffolds. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 521–550. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkifli, F.H.; Jahir Hussain, F.S.; Abdull Rasad, M.S.B.; Mohd Yusoff, M. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym. Degrad. Stab. 2014, 110, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; He, R.; Sha, B.; Li, W.; Qing, H.; Teng, R.; Xu, F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2018, 92, 995–1005. [Google Scholar] [CrossRef]
- Lee, W.Y.-w.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Transl. 2017, 9, 76–88. [Google Scholar] [CrossRef]
- Yuan, X.; Mak, A.F.T.; Kwok, K.W.; Yung, B.K.O.; Yao, K. Characterization of poly(L-lactic acid) fibers produced by melt spinning. J. Appl. Polym. Sci. 2001, 81, 251–260. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable Surface Modification of Poly(lactic-co-glycolic acid) (PLGA) by Hydrolysis or Aminolysis I: Physical, Chemical, and Theoretical Aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Haider, A.; Choi, Y.-r.; Kang, I.-k. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Liu, D.; Kuang, H.; Cai, J.; Chen, W.; Sun, B.; Xia, L.; Fang, B.; Morsi, Y.; Mo, X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2019, 534, 625–636. [Google Scholar] [CrossRef]
- Wu, J.; Xie, L.; Lin, W.Z.Y.; Chen, Q. Biomimetic nanofibrous scaffolds for neural tissue engineering and drug development. Drug Discov. Today 2017, 22, 1375–1384. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Uma Maheshwari, S.; Samuel, V.K.; Nagiah, N. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Binulal, N.S.; Selvamurugan, N.; Nair, S.V.; Menon, D.; Furuike, T.; Tamura, H.; Jayakumar, R. Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 77, 863–869. [Google Scholar] [CrossRef]
- Chahal, S.; Hussain, F.S.J.; Yusoff, M.B.M. Characterization of Modified Cellulose (MC)/Poly (Vinyl Alcohol) Electrospun Nanofibers for Bone Tissue Engineering. Procedia Eng. 2013, 53, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Hussain, F.S.J.; Kumar, A.; Rasad, M.S.B.A.; Yusoff, M.M. Fabrication, characterization and in vitro biocompatibility of electrospun hydroxyethyl cellulose/poly (vinyl) alcohol nanofibrous composite biomaterial for bone tissue engineering. Chem. Eng. Sci. 2016, 144, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Jahir Hussain, F.S.; Kumar, A.; Yusoff, M.M.; Bahari Abdull Rasad, M.S. Electrospun hydroxyethyl cellulose nanofibers functionalized with calcium phosphate coating for bone tissue engineering. Rsc Adv. 2015, 5, 29497–29504. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Hussain, F.S.J.; Yusoff, M.M.; Abdull Rasad, M.S.B.; Kumar, A. Nanohydroxyapatite-coated hydroxyethyl cellulose/poly (vinyl) alcohol electrospun scaffolds and their cellular response. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 115–122. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Barakat, N.A.M.; Kanjwal, M.A.; Park, S.J.; Park, D.K.; Kim, H.Y. Synthesis of poly(vinyl alcohol) (PVA) nanofibers incorporating hydroxyapatite nanoparticles as future implant materials. Macromol. Res. 2010, 18, 59–66. [Google Scholar] [CrossRef]
- Lee, J.; Deng, Y. Nanoindentation study of individual cellulose nanowhisker-reinforced PVA electrospun fiber. Polym. Bull. 2013, 70, 1205–1219. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kraft, D.C.E.; Harkness, L.; Melsen, B.; Varma, H.; Nair, P.D.; Kjems, J.; Kassem, M. Bioactive nano-fibrous scaffold for vascularized craniofacial bone regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1537–e1548. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Idris, A.; Irfan, M.; Kurniawan, D.; Yusof, N.M.; Nasiri, R. γ-Fe2O3 nanoparticles filled polyvinyl alcohol as potential biomaterial for tissue engineering scaffold. J. Mech. Behav. Biomed. Mater. 2015, 49, 90–104. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Yusof, N.M.; Idris, A.; Misran, E.; Kurniawan, D. Development of highly porous biodegradable γ-Fe2O3/polyvinyl alcohol nanofiber mats using electrospinning process for biomedical application. Mater. Sci. Eng. C 2017, 70, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Shankhwar, N.; Kumar, M.; Mandal, B.B.; Srinivasan, A. Novel polyvinyl alcohol-bioglass 45S5 based composite nanofibrous membranes as bone scaffolds. Mater. Sci. Eng. C 2016, 69, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Ma, X.; Li, J.; Cheng, Y.; Cao, Y.; Wang, Z.; Shi, X.; Du, Y.; Deng, H.; Li, Z. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int. J. Pharm. 2018, 547, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Kashef-Saberi, M.S.; Roodbari, N.H.; Parivar, K.; Vakilian, S.; Hanaee-Ahvaz, H. Enhanced Osteogenic Differentiation of Mesenchymal Stem Cells on Electrospun Polyethersulfone/Poly(Vinyl) Alcohol/Platelet Rich Plasma Nanofibrous Scaffolds. Asaio J. 2018, 64, 115–122. [Google Scholar]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- Zarekhalili, Z.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int. J. Biol. Macromol. 2017, 94, 679–690. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.-C.; Oh, G.-W.; Heo, S.-Y.; Nguyen, V.-T.; Jeon, Y.-J.; Lee, B.; Jang, C.H.; Kim, G.; Park, W.S.; et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int. J. Biol. Macromol. 2015, 81, 504–513. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Rasad, M.S.B.A.; Mohd Yusoff, M. Nanostructured materials from hydroxyethyl cellulose for skin tissue engineering. Carbohydr. Polym. 2014, 114, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Vashisth, P.; Nikhil, K.; Roy, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. A novel gellan–PVA nanofibrous scaffold for skin tissue regeneration: Fabrication and characterization. Carbohydr. Polym. 2016, 136, 851–859. [Google Scholar] [CrossRef]
- Mihov, D.; Spiess, M. Glycosaminoglycans: Sorting determinants in intracellular protein traffic. Int. J. Biochem. Cell Biol. 2015, 68, 87–91. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly(vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Vaishya, R. The journey of articular cartilage repair. JCOT 2016, 7, 135–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, A.; Dennis, J.E.; Awadallah, A.; Carrino, D.A.; Mansour, J.M.; Kastenbauer, E.; Caplan, A.I. Immunochemical and Mechanical Characterization of Cartilage Subtypes in Rabbit. J. Histochem. Cytochem. 2002, 50, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-Y.; Nukavarapu, S. 11-Scaffolds for cartilage tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume One; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 211–244. [Google Scholar]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.R.; Felson, D.T. Updated Estimates Suggest a Much Higher Prevalence of Arthritis in United States Adults Than Previous Ones. Arthritis Rheumatol. 2018, 70, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Tompson, S.W.; Merriman, B.; Funari, V.A.; Fresquet, M.; Lachman, R.S.; Rimoin, D.L.; Nelson, S.F.; Briggs, M.D.; Cohn, D.H.; Krakow, D. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am. J. Hum. Genet. 2009, 84, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, G.; Cao, Y. Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Kazemnejad, S.; Khanmohammadi, M.; Baheiraei, N.; Arasteh, S. Current State of Cartilage Tissue Engineering using Nanofibrous Scaffolds and Stem Cells. AJMB 2017, 9, 50–65. [Google Scholar]
- Zhao, G.; Yin, S.; Liu, G.; Cen, L.; Sun, J.; Zhou, H.; Liu, W.; Cui, L.; Cao, Y. In vitro engineering of fibrocartilage using CDMP1 induced dermal fibroblasts and polyglycolide. Biomaterials 2009, 30, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Oktay, B.; Kayaman-Apohan, N.; Erdem-Kuruca, S.; Süleymanoğlu, M. Fabrication of collagen immobilized electrospun poly (vinyl alcohol) scaffolds. Polym. Adv. Technol. 2015, 26, 978–987. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Tsai, W.-C.; Chang, S.-H. Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J. Biomater. Sci. Polym. Ed. 2017, 28, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Anarkoli, A.O.; Rafienia, M.; Ghasemi, N.; Davary, N.; Bonakdar, S.; Naeimi, M.; Agheb, M.; Salamat, M.R. Incorporation of zeolite and silica nanoparticles into electrospun PVA/collagen nanofibrous scaffolds: The influence on the physical, chemical properties and cell behavior. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 457–465. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Sathyamurthy, M.; Lee, G.M.; Park, S.B. Electrospun chitosan/poly(vinyl alcohol) reinforced with CaCO3 nanoparticles with enhanced mechanical properties and biocompatibility for cartilage tissue engineering. Compos. Sci. Technol. 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Hung, S.-C.; Lai, J.-Y.; Wang, D.-M.; Hsieh, H.-J. Electrospun chitosan–gelatin–polyvinyl alcohol hybrid nanofibrous mats: Production and characterization. J. Taiwan Inst. Chem. Eng. 2014, 45, 1975–1981. [Google Scholar] [CrossRef]
- Sadeghi, A.; Pezeshki-Modaress, M.; Zandi, M. Electrospun polyvinyl alcohol/gelatin/chondroitin sulfate nanofibrous scaffold: Fabrication and in vitro evaluation. Int. J. Biol. Macromol. 2018, 114, 1248–1256. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, W.; Hao, H.; Li, J.; Luo, F.; Tan, H. Nanofibrous scaffold from electrospinning biodegradable waterborne polyurethane/poly(vinyl alcohol) for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2017, 28, 648–663. [Google Scholar] [CrossRef]
- Pillai, M.M.; Gopinathan, J.; Indumathi, B.; Manjoosha, Y.R.; Santosh Sahanand, K.; Dinakar Rai, B.K.; Selvakumar, R.; Bhattacharyya, A. Silk–PVA Hybrid Nanofibrous Scaffolds for Enhanced Primary Human Meniscal Cell Proliferation. J. Membr. Biol. 2016, 249, 813–822. [Google Scholar] [CrossRef]
- Parikh, V.; Kadiwala, J.; Hidalgo Bastida, A.; Holt, C.; Sanami, M.; Miraftab, M.; Shakur, R.; Azzawi, M. Small diameter helical vascular scaffolds support endothelial cell survival. Nanomed. Nanotechnol. 2018, 14, 2598–2608. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, H.; Gao, X.; Liu, T.; Tan, Y. Composite vascular grafts with high cell infiltration by co-electrospinning. Mater. Sci. Eng. C 2016, 67, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Shinoka, T.; Duncan, D.; Hibino, N.; Solomon, D.; Cleary, M.; Rathore, A.; Fein, C.; Church, S.; Breuer, C. Vascular tissue engineering: Towards the next generation vascular grafts. Adv. Drug Deliv. Rev. 2011, 63, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Lv, M.; Shi, Y.; Cao, D. Super hydrophilic poly(ethylene terephthalate) (PET)/poly(vinyl alcohol) (PVA) composite fibrous mats with improved mechanical properties prepared via electrospinning process. Colloids Surf. A 2013, 436, 417–424. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ramakrishna, S. Strategies for regeneration of components of nervous system: Scaffolds, cells and biomolecules. Regen. Biomater. 2015, 2, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Kaucká, M.; Adameyko, I. Non-canonical functions of the peripheral nerve. Exp. Cell Res. 2014, 321, 17–24. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [Green Version]
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Mottaghitalab, V.; Ziabari, M.; Divsalar, A.; Shokrgozar, M.A. Enhancement of neural cell lines proliferation using nano-structured chitosan/poly(vinyl alcohol) scaffolds conjugated with nerve growth factor. Carbohydr. Polym. 2011, 86, 526–535. [Google Scholar] [CrossRef]
- Shokrgozar, M.A.; Mottaghitalab, F.; Mottaghitalab, V.; Farokhi, M. Fabrication of porous chitosan/poly(vinyl alcohol) reinforced single-walled carbon nanotube nanocomposites for neural tissue engineering. J. Biomed. Nanotechnol. 2011, 7, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Esmaeili, F.; Katouli, S.N. Fabrication of Chitosan/Poly (vinyl alcohol)/Carbon Nanotube/Bioactive Glass Nanocomposite Scaffolds for Neural Tissue Engineering. J. Nanomed. Res. 2016, 4, 00088. [Google Scholar]
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; El Haj, A.J.; Yang, Y. Chemical and Topographical Effects on Cell Differentiation and Matrix Elasticity in a Corneal Stromal Layer Model. Adv. Funct. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Mandal, B.B.; Park, S.-H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, C.R.; Tsai, R.J.; Latorre, M.A.; Griffith, M. Bioengineered corneas for transplantation and in vitro toxicology. Front. Biosci. 2009, 1, 3326–3337. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Kobayashi, H. Surface Modified Poly(vinyl alcohol) Nanofiber for the Artificial Corneal Stroma. Key Eng. Mater. 2007, 342, 209–212. [Google Scholar] [CrossRef]
- Seyed, M.A.; Vijayaraghavan, K. Evaluation of an Improved Chitosan Scaffold Cross-Linked With Polyvinyl Alcohol and Amine Coupling Through 1-Ethyl-3-(3-Dimethyl Aminopropyl)-Carbodiimide (EDC) and 2 N-Hydroxysuccinimide (NHS) for Corneal Applications. Maced. J. Med. Sci. 2018, 6, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, H.F.; Luqman, M.; Khalil, K.A.; Elnakady, Y.A.; Abd-Elkader, O.H.; Rady, A.M.; Alharthi, N.H.; Karim, M.R. Fabrication of core-shell structured nanofibers of poly (lactic acid) and poly (vinyl alcohol) by coaxial electrospinning for tissue engineering. Eur. Polym. J. 2018, 98, 483–491. [Google Scholar] [CrossRef]
- Enderami, S.E.; Kehtari, M.; Abazari, M.F.; Ghoraeian, P.; Nouri Aleagha, M.; Soleimanifar, F.; Soleimani, M.; Mortazavi, Y.; Nadri, S.; Mostafavi, H.; et al. Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Increasing | Decreasing | |
|---|---|---|
| Molecular Weight | Viscosity | Flexibility |
| Tensile Strength | Water Sensitivity | |
| Water Resistance | Ease of Solvation | |
| Solvent Resistance | ||
| Dispersing Power | ||
| Adhesive Strength | ||
| Block Resistance | ||
| Percentage of Hydrolysis | Tensile Strength | Flexibility |
| Block Resistance | Dispersing Power | |
| Water Resistance | Water Sensitivity | |
| Solvent Resistance | Adhesion to Hydrophobic Surfaces | |
| Adhesion to Hydrophilic Surfaces |
| Crosslinker | Structure | Functionality | Applications |
|---|---|---|---|
| Glutaraldehyde | Aliphatic dialdehyde | Two–CHO groups | Reducing oxygen permeability. |
| Drug delivery applications | |||
| Proton exchange membrane | |||
| Ultrafiltration membranes | |||
| Pervaporation systems | |||
| Coating | |||
| Maleic acid | Aliphatic dicarboxylic acids | Two–COOH groups | Separation processes |
| Fumaric acid | Pervaporation systems | ||
| Malic acid | Pervaporation systems | ||
| Sulfosuccinic acid | Proton and methanol transport | ||
| Phthalic acid | Aromatic dicarboxylic acids | Development of polysulfone (PS) membranes | |
| Iso-phthalic acid | |||
| Terephthalic acid | |||
| Aconitic acid (cis and trans) | Aliphatic tricarboxylic acids | Three–COOH groups | Development of polysulfone membranes |
| Citric acid | Support membrane for polysulfone | ||
| Hexamethylene diisocyanate | Aliphatic diisocyanate | Two–NCO groups | Improved thermal and mechanical properties of PVA |
| Boric acid | Non-linear | Three–OH groups | Improved melting behavior of PVA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010007
Teixeira MA, Amorim MTP, Felgueiras HP. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers. 2020; 12(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010007
Chicago/Turabian StyleTeixeira, Marta A., M. Teresa P. Amorim, and Helena P. Felgueiras. 2020. "Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications" Polymers 12, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010007