Selective and Colorimetric Detection of p-Nitrophenol Based on Inverse Opal Polymeric Photonic Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fabrication of Photonic Crystal Templates
2.3. Preparation of Inverse Opal Polymeric Photonic Crystals
2.4. Characterization
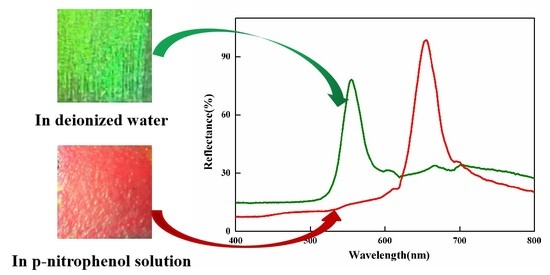
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, K.; Yang, Y.; Zhu, Y.; Zhang, J. Highly selective self-powered sensing platform for p-nitrophenol detection constructed with a photocathode-based photocatalytic fuel cell. Anal. Chem. 2017, 89, 8599–8603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, S.; Palladino, P.; Pascale, E.; Brittoli, A.; Minunni, M. Colorimetric determination of p-nitrophenol by using elisa microwells modified with an adhesive polydopamine nanofilm containing catalytically active gold nanoparticles. Microchim. Acta 2019, 186, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, K.; Kumar, M.; Chauhan, M.S. Solution combustion synthesis of CeO2/ZnO nano-composite as a potential scaffold for detection and degradation of p-nitrophenol. Mater. Chem. Phys. 2019, 226, 59–65. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.; Mo, S.; Li, N.; Ju, Y.; Ling, Y.; Li, N.; Luo, H. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Li, Y.; Li, C. Electrochemical simultaneous determination of hydroquinone and p-nitrophenol based on host-guest molecular recognition capability of dual β-cyclodextrin functionalized Au@graphene nanohybrids. Sens. Actuators B: Chem. 2015, 207, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, Z.; Zeng, Y.; Zhou, T.; Shi, G. A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens. Bioelectron. 2014, 52, 317–323. [Google Scholar] [CrossRef]
- Oliveira, R.; Santos, N.; Alves, L.; Lima, K.; Kubota, L.; Damos, F.; Luz, R. Highly sensitive pnitrophenol determination employing a new sensor based on N-Methylphenazonium methyl sulfate and graphene: Analysis in natural and treated waters. Sens. Actuators B: Chem. 2015, 221, 740–749. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Su, D.; Xia, Q.; Chai, F.; Wang, C.; Qu, F. Fluorescent detection of TNT and 4-nitrophenol by BSA Au nanoclusters. Dalton. Trans. 2014, 43, 10057–10063. [Google Scholar] [CrossRef]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [Green Version]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Wu, Q.; Lu, Y.; Wang, R.; Zhang, Q.; Qi, J.; Yao, J.; Xu, J. Polarization-resolved edge states in terahertz topological photonic crystal. Opt. Express 2019, 27, 22819–22826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ding, T. Micropatterning and defect engineering of colloidal photonic crystals via laser direct writing. J. Mater. Chem. C 2019, 7, 13410–13414. [Google Scholar] [CrossRef]
- Chi, J.; Shao, C.; Du, X.; Liu, H.; Gu, Z. Generating microdroplet array on photonic pseudo-paper for absolute quantification of nucleic acids. ACS Appl. Mater. Interfaces 2018, 10, 39144–39150. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Niu, W.; Qu, L.; Zhang, S. Two-way rewritable and stable photonic patterns enabled by near-infrared laser-responsive shape memory photonic crystals. J. Mater. Chem. C 2019, 7, 1896–1903. [Google Scholar] [CrossRef]
- Ding, T.; Valev, V.K.; Salmon, A.R.; Forman, C.J.; Smoukov, S.K.; Scherman, O.A.; Frenkel, D.; Baumberg, J.J. Light-induced actuating nanotransducers. Proc. Natl. Acad. Sci. USA 2016, 113, 5503–5507. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Mertens, J.; Sigle, D.O.; Baumberg, J. Capillary-Force-Assisted Optical Tuning of Coupled Plasmons. Adv. Mater. 2015, 27, 6457–6461. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Goponenko, A.V.; Asher, S.A. Polymerized polyHEMA photonic crystals: pH and ethanol sensor materials. J. Am. Chem. Soc. 2008, 130, 3113–3119. [Google Scholar] [CrossRef]
- Stawska, H.I.; Popenda, M.A.; Bereś-Pawlik, E. Anti-resonant hollow core fibers with modified shape of the core for the better optical performance in the Visible Spectral Region—A Numerical Study. Polymers 2018, 10, 899. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Fu, Q.; Chen, K.; Ge, J. Liquid photonic crystals for mesopore detection. Angew. Chem. Int. Ed. 2018, 57, 252–256. [Google Scholar] [CrossRef]
- Fang, Y.; Ni, Y.; Leo, S.-Y.; Taylor, C.; Basile, V.; Jiang, P. Reconfigurable photonic crystals enabled by pressure-responsive shape-memory polymers. Nat. Commun. 2015, 6, 7416. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, K.; Huang, Y.-S.; Huang, B.-R.; Huang, C.-F.; Chen, J.-K. Real-Time Packing behavior of core-shell silica@poly(N-isopropylacrylamide) microspheres as photonic crystals for visualizing in thermal sensing. Polymers 2016, 8, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zeng, F.; Pang, F.; Ge, J. Substantial enhancement toward the photocatalytic activity of CdS quantum dots by photonic crystal-supporting films. ACS Appl. Mater. Interfaces 2018, 10, 42241–42248. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Lyu, Q.; Xie, Z.; Li, M.; Wang, K.; Xiong, B.; Zhang, L.; Zhu, J. Metallosupramolecular photonic elastomers with self-healing capability and angle-independent color. Adv. Mater. 2018, 31, 1805496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Htein, L.; Gunawardena, D.S.; Chung, W.-H.; Lu, C.; Lee, K.-K.; Tam, H.-Y. Novel accelerometer realized by a polarization-maintaining photonic crystal fiber for railway monitoring applications. Opt. Express 2019, 27, 21597–21607. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xia, H.; Xu, J.; Sun, X.; Liu, X. Manipulating luminescence of light emitters by photonic crystals. Adv. Mater. 2018, 30, 1803362. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.; Liu, J.; Li, Y.; Wu, M.; Van Tendeloo, G.; Su, B. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Y. Stimuli-responsive optical nanomaterials. Adv. Mater. 2019, 31, 1807061. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A gelated colloidal crystal attached lens for noninvasive continuous monitoring of tear glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H. A bioinspired color-changing polystyrene microarray as a rapid qualitative sensor for methanol and ethanol. Mater. Chem. Phys. 2016, 173, 285–290. [Google Scholar] [CrossRef]
- Kuo, W.-K.; Hsu, J.-J.; Nien, C.-K.; Yu, H. Moth-eye-inspired biophotonic surfaces with antireflective and hydrophobic characteristics. ACS Appl. Mater. Interfaces 2016, 8, 32021–32030. [Google Scholar] [CrossRef]
- Nien, C.-K.; Yu, H. The applications of biomimetic cicada-wing structure on the organic light-emitting diodes. Mater. Chem. Phys. 2019, 227, 191–199. [Google Scholar] [CrossRef]
- Li, L.; Zhao, B.; Long, Y.; Gao, J.-M.; Yang, G.; Tung, C.-H.; Song, K. Visual detection of carbonate ions by inverse opal photonic crystal polymers in aqueous solution. J. Mater. Chem. C 2015, 3, 9524–9527. [Google Scholar] [CrossRef]
- Li, L.; Long, Y.; Gao, J.-M.; Song, K.; Yang, G. Label-free and pH-sensitive colorimetric materials for the sensing of urea. Nanoscale 2016, 8, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Meng, Z.; Wang, Y.; Huang, S.; Wang, Q.; Lu, W.; Xue, M. A molecularly imprinted colloidal array as a colorimetric sensor for label-free detection of p-nitrophenol. Anal. Methods 2014, 6, 831–837. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Sigitov, V. Swelling, shrinking, deformation, and oscillation of polyampholyte gels based on vinyl 2-aminoethyl ether and sodium acrylate. Langmuir 1999, 15, 4230–4235. [Google Scholar] [CrossRef]
- Gurge, R.; Sarker, A.; Lahti, P.; Hu, B.; Karasz, F. Light emitting properties of fluorine-substituted poly(1,4-phenylene vinylenes). Macromolecules 1997, 30, 8286–8292. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Meng, T.; Zhang, W.; Su, Y.; Wei, J.; Shi, X.; Zhang, G. Selective and Colorimetric Detection of p-Nitrophenol Based on Inverse Opal Polymeric Photonic Crystals. Polymers 2020, 12, 83. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010083
Li L, Meng T, Zhang W, Su Y, Wei J, Shi X, Zhang G. Selective and Colorimetric Detection of p-Nitrophenol Based on Inverse Opal Polymeric Photonic Crystals. Polymers. 2020; 12(1):83. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010083
Chicago/Turabian StyleLi, Lu, Tiantian Meng, Wanbin Zhang, Ying Su, Juan Wei, Xinwei Shi, and Guanghua Zhang. 2020. "Selective and Colorimetric Detection of p-Nitrophenol Based on Inverse Opal Polymeric Photonic Crystals" Polymers 12, no. 1: 83. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010083