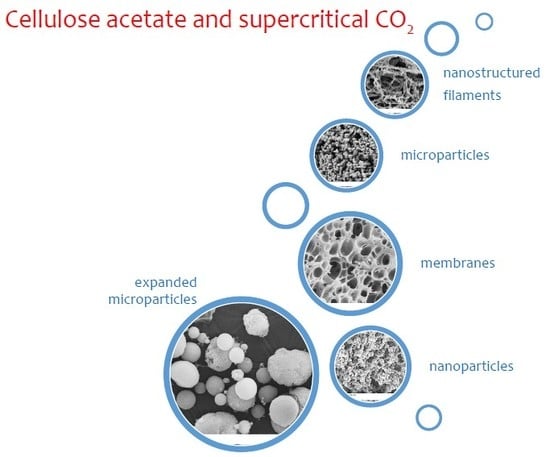
Cellulose Acetate and Supercritical Carbon Dioxide: Membranes, Nanoparticles, Microparticles and Nanostructured Filaments
Abstract
:1. Introduction
- the spray drying process is characterized by variations in particle shape and particle size distribution, high process temperatures, and high drying speeds that normally do not allow the encapsulation of temperature-sensitive bioactive substances [6];
- the electrospinning process is characterized by the use of organic solvents that can be toxic, difficulty in obtaining 3-D structures as well as sufficient size of pores needed for biomedical applications and suitable mechanical behavior; moreover, the process depends on a high number of variables and their combination (humidity, temperatures, collector distance, high voltage, etc.) [7];
- the chemical foaming process is simple, but high mold manufacturing precision is required (the mold cost is high), and a second clamping pressure device is needed during high-pressure foaming process [8];
- the gel drying process is characterized by a high cost of the raw materials (the chemicals) and there is often a large volume shrinkage and cracking during the drying step (the removing of the “organics” can cause the collapse of the structure due to their surface tension); moreover, long processing times can be necessary [9];
- the phase inversion process is characterized by the use of organic solvents and necessity of post-treatments on the generated material, low versatility (once the system polymer-solvent-nonsolvent is selected, only one kind of morphology is possible), long processing times (from several hours to days), etc. [5].
- attainment of a discontinuous structure (micro and nanoparticles, for example);
- attainment of a continuous structure with discontinuities (such as fibers, membranes, foams, and scaffolds).
- in both of them, the carbon dioxide acts as an antisolvent and flows continuously into the vessel;
- the techniques are both in a batch mode with respect to the solid phase (a depressurization is necessary to recover the solute after the process);
- the SAS process is continuous with respect to the polymeric solution that is injected through a nozzle in the supercritical medium, whereas, the scCO2 assisted phase inversion is batch, considering that the polymeric solution is charged at atmospheric pressure at the beginning of the experiment.
2. Apparatus, Materials and Methods
2.1. Materials
2.2. Apparatus and Procedures
2.2.1. Apparatus
2.2.2. Procedure for SAS Experiments
2.2.3. Procedure for Membranes’ Preparation
2.3. Characterization
3. Results and Discussion
3.1. Supercritical Antisolvent Precipitation
3.1.1. AC as the Liquid Solvent
3.1.2. AC/DMSO as the Liquid Solvent
3.2. Supercritical Phase Inversion Experiments
- (a)
- starting from a high polymer concentration solution, the demixing point will be located in the upper part of the miscibility hole between the binodal and spinodal curves (point A in Figure 5a) and a liquid-liquid binodal demixing with nucleation and growth of the polymer-lean phase inside the polymer-rich phase occurs, generating a cellular structure membrane (CM). An examplificative FESEM image is reported in Figure 6a;
- (b)
- decreasing the polymer concentration of the starting solution, the phase inversion occurs in the central part of the demixing hole of the ternary diagram in which the demixing point is located inside the spinodal curve (point B in Figure 5a), where a spinodal demixing is favored, leading to the generation of a spinodal membrane morphology (SM). An example of this morphology is reported in Figure 6b;
- (c)
- starting from low polymer concentration solutions, the demixing point is located in the lower part of the miscibility hole, again between the binodal and spinodal curves (point C in Figure 5a); a liquid-liquid binodal demixing with nucleation and growth of the polymer-rich phase inside the polymer-lean phase is favored, leading to the formation of a bead-like membrane (BLM). An examplificative FESEM image is reported in Figure 6c.
4. Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beck-Broichsitter, M.; Schweiger, C.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 2012, 158, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Thomas, N.L. Fabrication of porous fibers via electrospinning: Strategies and applications. Polym. Rev. 2019. [Google Scholar] [CrossRef]
- Gong, W.; Fu, H.; Zhang, C.; Ban, D.; Yin, X.; He, Y.; He, L.; Pei, X. Study on Foaming Quality and Impact Property of Foamed Polypropylene Composites. Polymers 2018, 10, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Van de Witte, P.; Dijkstra, P.; Van den Berg, J.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, M.; Gu, X. Seaweed-Derived Hydrocolloids as Food Coating and Encapsulation Agents. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–175. [Google Scholar]
- Kalluri, S.; Seng, K.H.; Guo, Z.; Liu, H.K.; Dou, S.X. Electrospun lithium metal oxide cathode materials for lithium-ion batteries. RSC Adv. 2013, 3, 25576–25601. [Google Scholar] [CrossRef]
- Jin, F.-L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.B.; Norton, M.G. Sols, gels, and Organic Chemistry. In Ceramic Materials; Springer: New York, NY, USA, 2013; pp. 411–422. [Google Scholar]
- Yeo, S.-D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Garay, I.; Pocheville, A.; Madariaga, L. Polymeric microparticles prepared by supercritical antisolvent precipitation. Powder Technol. 2010, 197, 211–217. [Google Scholar] [CrossRef]
- Cardea, S.; Baldino, L.; Pisanti, P.; Reverchon, E. 3-D PLLA scaffolds formation by a supercritical freeze extraction assisted process. J. Mater. Sci. Mater. 2014, 25, 355–362. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; Reverchon, E. Interpenetration of natural polymer aerogels by supercritical drying. Polymers 2016, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Q.; Xing, Z.; Zhang, W.; Zhang, M.; Tan, H.; Wang, J.; Wu, G. Improving the supercritical CO2 foaming of polypropylene by the addition of fluoroelastomer as a nucleation agent. Polymers 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; Martínez de la Ossa, E.J. Polymer and ampicillin co-precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2012, 63, 92–98. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Montes, A.; Merino, R.; De Los Santos, D.M.; Pereyra, C.; Martínez De La Ossa, E.J. Micronization of vanillin by rapid expansion of supercritical solutions process. J. CO2 Util. 2017, 21, 169–176. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Util. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Barrett, A.M.; Dehghani, F.; Foster, N.R. Increasing the Dissolution Rate of Itraconazole Processed by Gas Antisolvent Techniques using Polyethylene Glycol as a Carrier. Pharm. Res. 2007, 25, 1274–1289. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Martín, Á.; Pham, H.M.; Kilzer, A.; Kareth, S.; Weidner, E. Micronization of polyethylene glycol by PGSS (Particles from Gas Saturated Solutions)-drying of aqueous solutions. Chem. Eng. Process. 2010, 49, 1259–1266. [Google Scholar] [CrossRef]
- Della Porta, G.; Campardelli, R.; Falco, N.; Reverchon, E. PLGA microdevices for retinoids sustained release produced by supercritical emulsion extraction: Continuous versus batch operation layouts. J. Pharm. Sci. 2011, 100, 4357–4367. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Nanostructured cellulose acetate filaments produced by supercritical antisolvent precipitation. J. Supercrit. Fluids 2011, 55, 1095–1103. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S.; Rapuano, C. Formation of poly-vinyl-alcohol structures by supercritical CO2. J. Appl. Pol. Sci. 2007, 104, 3151–3160. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk fibroin aerogels: Potential scaffolds for tissue engineering applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2007, 14, 49–64. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Formation of cellulose acetate membranes using a supercritical fluid assisted process. J. Membr. Sci. 2004, 240, 187–195. [Google Scholar] [CrossRef]
- Martson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. Pol. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.P.; Chandiran, I.S.; Jayaveera, K.N. Formulation and evaluation of glimepiride loaded cellulose acetate microparticles. Int. J. Pharm. Pharm. Sci. 2014, 6, 511–515. [Google Scholar]
- De Marco, I.; Prosapio, V.; Cice, F.; Reverchon, E. Use of solvent mixtures in supercritical antisolvent process to modify precipitates morphology: Cellulose acetate microparticles. J. Supercrit. Fluids 2013, 83, 153–160. [Google Scholar] [CrossRef]
- Kulterer, M.R.; Reischl, M.; Reichel, V.E.; Hribernik, S.; Wu, M.; Kostler, S.; Kargl, R.; Ribitsch, V. Nanoprecipitation of cellulose acetate using solvent/nonsolvent mixtures as dispersive media. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 23–29. [Google Scholar] [CrossRef]
- XXXI United States Pharmacopeia. General Chapter <467>, Organic Volatile Impurities; XXXI United States Pharmacopeia: Rockville, MD, USA, 2007. [Google Scholar]
- Campardelli, R.; Reverchon, E.; De Marco, I. Dependence of SAS particle morphologies on the ternary phase equilibria. J. Supercrit. Fluids 2017, 130 (Suppl. C), 273–281. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. PVP microparticles precipitation from acetone-ethanol mixtures using SAS process: Effect of phase behavior. J. Supercrit. Fluids 2019, 143, 321–329. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- De Marco, I.; Knauer, O.; Cice, F.; Braeuer, A.; Reverchon, E. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization: The influence of solvents. Chem. Eng. J. 2012, 203, 71–80. [Google Scholar] [CrossRef]







| # | c, mg/mL | P, MPa | T, °C | Morph. | m.d. ± s.d., nm | Figure |
|---|---|---|---|---|---|---|
| AC as the liquid solvent | ||||||
| 1 | 10 | 9 | 40 | NF | 92 ± 22 | |
| 2 | 12 | 35 | NF | 78 ± 18 | Figure 1a,b | |
| 3 | 40 | NF | 80 ± 20 | |||
| 4 | 50 | NF | 108 ± 21 | |||
| 5 | 15 | 35 | NP | 76 ± 14 | ||
| 6 | 40 | NP | 78 ± 15 | |||
| 7 | 50 | NP | 82 ± 19 | |||
| 8 | 15 | 9 | 60 | EMP | 16,070 ± 6170 | |
| 9 | 12 | 35 | NF | 100 ± 22 | ||
| 10 | 40 | NF | 108 ± 24 | |||
| 11 | 50 | NF | 110 ± 24 | |||
| 12 | 60 | NF | 114 ± 26 | |||
| 13 | 15 | 35 | NP | 78 ± 14 | ||
| 14 | 50 | NP | 80 ± 15 | |||
| 15 | 60 | NP | 83 ± 16 | Figure 2 | ||
| 16 | 20 | 9 | 60 | EMP | 30,600 ± 15,230 | Figure 3 |
| 17 | 12 | 40 | NF | 109 ± 25 | ||
| 18 | 15 | 40 | NF | 102 ± 22 | ||
| 19 | 30 | 9 | 60 | EMP | 31,300 ± 16,200 | |
| 20 | 12 | 40 | NF | 132 ± 28 | ||
| 21 | 15 | 40 | NF | 112 ± 23 | ||
| AC/DMSO 50/50 as the liquid solvent | ||||||
| 22 | 20 | 9 | 40 | MP | 232 ± 50 | |
| 23 | 40 | MP | 403 ± 160 | Figure 4 | ||
| 24 | 60 | MP | 670 ± 60 | |||
| # | c, mg/mL | P, MPa | T, °C | Morph. | Figure |
|---|---|---|---|---|---|
| 25 | 40 | 8 | 45 | BLM | Figure 6c |
| 26 | 10 | BLM | |||
| 27 | 20 | BLM | |||
| 28 | 80 | 8 | 45 | SM | Figure 6b |
| 29 | 10 | SM | |||
| 30 | 20 | SM | |||
| 31 | 160 | 8 | 45 | CM | |
| 32 | 10 | CM | |||
| 33 | 20 | SM | |||
| 34 | 240 | 8 | 45 | CM | Figure 6a |
| 35 | 10 | CM | |||
| 36 | 320 | 10 | 45 | CM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardea, S.; De Marco, I. Cellulose Acetate and Supercritical Carbon Dioxide: Membranes, Nanoparticles, Microparticles and Nanostructured Filaments. Polymers 2020, 12, 162. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010162
Cardea S, De Marco I. Cellulose Acetate and Supercritical Carbon Dioxide: Membranes, Nanoparticles, Microparticles and Nanostructured Filaments. Polymers. 2020; 12(1):162. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010162
Chicago/Turabian StyleCardea, Stefano, and Iolanda De Marco. 2020. "Cellulose Acetate and Supercritical Carbon Dioxide: Membranes, Nanoparticles, Microparticles and Nanostructured Filaments" Polymers 12, no. 1: 162. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010162