The Effect of Poly(ethylene glycol) (PEG) Length on the Wettability and Surface Chemistry of PEG-Fluoroalkyl-Modified Polystyrene Diblock Copolymers and Their Two-Layer Films with Elastomer Matrix
Abstract
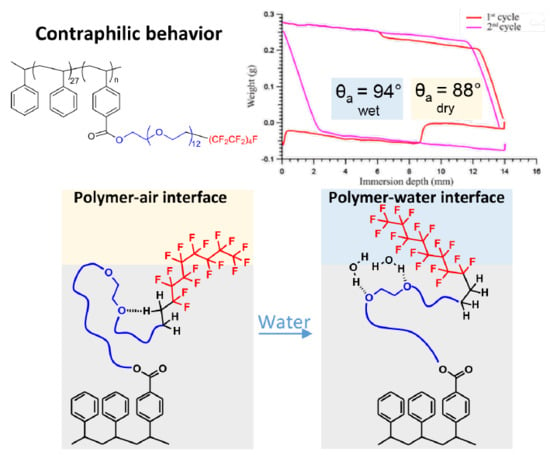
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.2.1. Synthesis of Polystyrene Macroinitiator
2.2.2. Synthesis of S27SzAn Diblock Copolymers
2.3. Deposition of One-Layer Films
2.4. Deposition of SEBS-Based Two-Layer Films
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of Block Copolymers
3.2. Wettability of Block Copolymer Films
3.2.1. Dynamic Contact Angles
3.2.2. Static Contact Angles
3.3. Preparation of Two-Layer Films
3.4. Wettability and Surface Tension of Two-Layer Films
3.5. Surface Composition of Two-Layer Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guazzelli, E.; Galli, G.; Martinelli, E.; Margaillan, A.; Bressy, C. Amphiphilic hydrolyzable polydimethylsiloxane-b-poly(ethyleneglycol methacrylate-co-trialkylsilyl methacrylate) block copolymers for marine coatings. I. Synthesis, hydrolysis and surface wettability. Polymer 2020, 186, 121954. [Google Scholar] [CrossRef]
- Gevaux, L.; Lejars, M.; Margaillan, A.; Briand, J.-F.; Bunet, R.; Bressy, C. Hydrolyzable additive-based silicone elastomers: A new approach for antifouling coatings. Polymers 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Rufin, M.A.; Ngo, B.K.D.; Barry, M.E.; Page, V.M.; Hawkins, M.L.; Stafslien, S.J.; Grunlan, M.A. Antifouling silicones based on surface-modifying additive amphiphiles. Green Mater. 2017, 5, 4–13. [Google Scholar] [CrossRef]
- Camós Noguer, A.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Diffusion of surface-active amphiphiles in silicone-based fouling-release coatings. Progress Org. Coat. 2017, 106, 77–86. [Google Scholar] [CrossRef]
- Yasani, B.R.; Martinelli, E.; Galli, G.; Glisenti, A.; Mieszkin, S.; Callow, M.E.; Callow, J.A. A comparison between different fouling-release elastomer coatings containing surface-active polymers. Biofouling 2014, 30, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Menghetti, S.; Galli, G.; Glisenti, A.; Krishnan, S.; Paik, M.Y.; Ober, C.K.; Smilgies, D.-M.; Fischer, D.A. Surface engineering of styrene/PEGylated-fluoroalkyl styrene block copolymer thin films. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 267–284. [Google Scholar] [CrossRef]
- Martinelli, E.; Agostini, S.; Galli, G.; Chiellini, E.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Graf, K.; Bartels, F.W. Nanostructured films of amphiphilic fluorinated block copolymers for fouling release application. Langmuir 2008, 24, 13138–13147. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Martinelli, E.; Galli, G.; Pretti, C. PDMS-based films containing surface-active amphiphilic block copolymers to combat fouling from barnacles B. amphitrite and B. improvisus. Polymer 2017, 108, 476–482. [Google Scholar] [CrossRef]
- Martinelli, E.; Pretti, C.; Oliva, M.; Glisenti, A.; Galli, G. Sol-gel polysiloxane films containing different surface-active trialkoxysilanes for the release of the marine foulant Ficopomatus enigmaticus. Polymer 2018, 145, 426–433. [Google Scholar] [CrossRef]
- Wanka, R.; Aldred, N.; Finlay, J.A.; Amuthalingam, A.; Clarke, J.L.; Clare, A.S.; Rosenhahn, A. antifouling properties of dendritic polyglycerols against marine macrofouling organisms. Langmuir 2019, 35, 16568–16575. [Google Scholar] [CrossRef]
- Grunlan, M.A.; Lee, N.S.; Cai, G.; Gädda, T.; Mabry, J.M.; Mansfeld, F.; Kus, E.; Wendt, D.E.; Kowalke, G.L.; Finlay, J.A.; et al. Synthesis of α,ω-bisepoxy oligo (1′H,1′H,2′H,2′H-perfluoroalkyl siloxane)s and properties of their photo-acid cross-linked films. Chem. Mater. 2004, 16, 2433–2441. [Google Scholar] [CrossRef]
- Wang, Y.; Finlay, J.A.; Betts, D.E.; Merkel, T.J.; Luft, J.C.; Callow, M.E.; Callow, J.A.; Desimone, J.M. Amphiphilic co-networks with moisture-induced surface segregation for high-performance nonfouling coatings. Langmuir 2011, 27, 10365–10369. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zeng, H.; Peng, Q.; Bressy, C.; Ma, C.; Zhang, G. Self-stratifying silicone coating with nonleaching antifoulant for marine anti-biofouling. Adv. Mater. Interfaces 2019, 6, 1900535. [Google Scholar] [CrossRef]
- Murthy, R.; Bailey, B.M.; Valentin-Rodriguez, C.; Ivanisevic, A.; Grunlan, M.A. Amphiphilic silicones prepared from branched PEO-silanes with siloxane tethers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4108–4119. [Google Scholar] [CrossRef]
- Ngo, B.K.D.; Barry, M.E.; Lim, K.K.; Johnson, J.C.; Luna, D.J.; Pandian, N.K.R.; Jain, A.; Grunlan, M.A. Thromboresistance of silicones modified with PEO-silane amphiphiles. ACS Biomater. Sci. Eng. 2020, 6, 2029–2037. [Google Scholar] [CrossRef]
- Martinelli, E.; Galli, G.; Cwikel, D.; Marmur, A. Wettability and surface tension of amphiphilic polymer films: Time-dependent measurements of the most stable contact angle. Macromol. Chem. Phys. 2012, 213, 1448–1456. [Google Scholar] [CrossRef]
- Galli, G.; Martinelli, E. Amphiphilic polymer platforms: Surface engineering of films for marine antibiofouling. Macromol. Rapid Commun. 2017, 38, 1600704. [Google Scholar] [CrossRef]
- Leonardi, A.K.; Ober, C.K. Polymer-based marine antifouling and fouling release surfaces: Strategies for synthesis and modification. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 241–264. [Google Scholar] [CrossRef]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Mielczarski, E.; Galli, G.; Morelli, A.; Martinelli, E.; Chiellini, E. The surface-segregated nanostructure of fluorinated copolymer-poly(dimethylsiloxane) blend films. Langmuir 2010, 26, 2871–2876. [Google Scholar] [CrossRef]
- Inutsuka, M.; Yamada, N.L.; Ito, K.; Yokoyama, H. High density polymer brush spontaneously formed by the segregation of amphiphilic diblock copolymers to the polymer/water interface. ACS Macro Lett. 2013, 2, 265–268. [Google Scholar] [CrossRef]
- Martinelli, E.; Fantoni, C.; Galli, G.; Gallot, B.; Glisenti, A. Low surface energy properties of smectic fluorinated block copolymer/SEBS blends. Mol. Cryst. Liq. Cryst. 2009, 500, 51–62. [Google Scholar] [CrossRef]
- Lee, H.; Archer, L.A. Functionalizing polymer surfaces by field-induced migration of copolymer additives. 1. Role of surface energy gradients. Macromolecules 2001, 34, 4572–4579. [Google Scholar] [CrossRef]
- Martinelli, E.; Hill, S.D.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Glisenti, A.; Galli, G. Amphiphilic modified-styrene copolymer films: Antifouling/fouling release properties against the green alga Ulva linza. Progress Org. Coat. 2016, 90, 235–242. [Google Scholar] [CrossRef]
- Martinelli, E.; Pelusio, G.; Yasani, B.R.; Glisenti, A.; Galli, G. surface chemistry of amphiphilic polysiloxane/triethyleneglycol-modified poly(pentafluorostyrene) block copolymer films before and after water immersion. Macromol. Chem. Phys. 2015, 216, 2086–2094. [Google Scholar] [CrossRef]
- Sorgi, C.; Martinelli, E.; Galli, G.; Pucci, A. Julolidine-labelled fluorinated block copolymers for the development of two-layer films with highly sensitive vapochromic response. Sci. China Chem. 2018, 61, 947–956. [Google Scholar] [CrossRef]
- Mackel, M.J.; Sanchez, S.; Kornfield, J.A. Humidity-dependent wetting properties of high hysteresis surfaces. Langmuir 2007, 23, 3–7. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Galli, G.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Hill, S. Amphiphilic pentablock copolymers and their blends with PDMS for antibiofouling coatings. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1213–1225. [Google Scholar] [CrossRef]
- Atlar, M.; Ünal, B.; Ünal, U.O.; Politis, G.; Martinelli, E.; Galli, G.; Davies, C.; Williams, D. An experimental investigation of the frictional drag characteristics of nanostructured and fluorinated fouling-release coatings using an axisymmetric body. Biofouling 2013, 29, 39–52. [Google Scholar] [CrossRef]
- Li, Y.; Chen, R.; Feng, Y.; Sun, X.; Tang, L.; Takahashi, K.; Liu, P.; Wang, J. Synthesis of amphiphilic acrylate boron fluorinated polymers with antifouling behavior. Ind. Eng. Chem. Res. 2019, 58, 8016–8025. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Guanghui, W.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-based fouling-release coatings for marine antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef] [Green Version]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Rufin, M.A.; Gruetzner, J.A.; Hurley, M.J.; Hawkins, M.L.; Raymond, E.S.; Raymond, J.E.; Grunlan, M.A. Enhancing the protein resistance of silicone via surface-restructuring PEO-silane amphiphiles with variable PEO length. J. Mater. Chem. B 2015, 3, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Miao, J.; Yan, J.; Yang, K.; Mao, C.; Ju, J.; Shen, J. Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood. Electrochim. Acta 2013, 89, 549–554. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Glisenti, A.; Galli, G. Surface segregation of amphiphilic PDMS-based films containing terpolymers with siloxane, fluorinated and ethoxylated side chains. Coatings 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Peng, H. Synthesis and application of fluorine-containing polymers with low surface energy. Polym. Rev. 2019, 59, 739–757. [Google Scholar]
- Martini, F.; Guazzelli, E.; Martinelli, E.; Borsacchi, S.; Geppi, M.; Galli, G. molecular dynamics of amphiphilic random copolymers in the bulk: A 1 H and 19 F NMR relaxometry study. Macromol. Chem. Phys. 2019, 1900177. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takenaka, M.; Sawamoto, M.; Terashima, T. Self-assembly of amphiphilic block pendant polymers as microphase separation materials and folded flower micelles. Polym. Chem. 2019. [Google Scholar] [CrossRef]
- Guazzelli, E.; Martinelli, E.; Galli, G.; Cupellini, L.; Jurinovich, S.; Mennucci, B. Single-chain self-folding in an amphiphilic copolymer: An integrated experimental and computational study. Polymer 2019, 161, 33–40. [Google Scholar] [CrossRef]
- Martinelli, E.; Guazzelli, E.; Galli, G.; Telling, M.T.F.; Poggetto, G.D.; Immirzi, B.; Domenici, F.; Paradossi, G. Prolate and temperature-responsive self-assemblies of amphiphilic random copolymers with perfluoroalkyl and polyoxyethylene side chains in solution. Macromol. Chem. Phys. 2018, 219, 1800210. [Google Scholar] [CrossRef]
- Martinelli, E.; Annunziata, L.; Guazzelli, E.; Pucci, A.; Biver, T.; Galli, G. The temperature-responsive nanoassemblies of amphiphilic random copolymers carrying poly(siloxane) and poly(oxyethylene) pendant chains. Macromol. Chem. Phys. 2018, 219, 1800082. [Google Scholar] [CrossRef]
- Terashima, T.; Sawamoto, M. Single-chain nanoparticles via self-folding amphiphilic copolymers in water. In Single-Chain Polymer Nanoparticles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 313–339. [Google Scholar]
- Ko, J.H.; Bhattacharya, A.; Terashima, T.; Sawamoto, M.; Maynard, H.D. Amphiphilic fluorous random copolymer self-assembly for encapsulation of a fluorinated agrochemical. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 352–359. [Google Scholar] [CrossRef]
- Zhang, W.; Fujiwara, T.; Taşkent, H.; Zheng, Y.; Brunson, K.; Gamble, L.; Wynne, K.J. A polyurethane surface modifier: Contrasting amphiphilic and contraphilic surfaces driven by block and random soft blocks having trifluoroethoxymethyl and PEG side chains. Macromol. Chem. Phys. 2012, 213, 1415–1434. [Google Scholar] [CrossRef]
- Horecha, M.; Senkovskyy, V.; Kiriy, A.; Stamm, M. Hydrophobically covered hydrogels: Preparation approaches and possible applications. In Progress in Colloid and Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 140, pp. 149–161. [Google Scholar]
- Makal, U.; Uslu, N.; Wynne, K.J. Water makes it hydrophobic: Contraphilic wetting for polyurethanes with soft blocks having semifluorinated and 5,5-dimethylhydantoin side chains. Langmuir 2007, 23, 209–216. [Google Scholar] [CrossRef]
- Makal, U.; Wynne, K.J. Water induced hydrophobic surface. Langmuir 2005, 21, 3742–3745. [Google Scholar] [CrossRef]
- Khongtong, S.; Ferguson, G.S. Integration of bulk and interfacial properties in a polymeric system: Rubber elasticity at a polybutadiene/water interface. J. Am. Chem. Soc. 2001, 123, 3588–3594. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Finlay, J.A.; Aldred, N.; Eller, M.J.; Felder, S.E.; Pollack, K.A.; Lonnecker, A.T.; Raymond, J.E.; MacKay, M.E.; Schweikert, E.A.; et al. Targeted surface nanocomplexity: Two-dimensional control over the composition, physical properties and anti-biofouling performance of hyperbranched fluoropolymer-poly(ethylene glycol) amphiphilic crosslinked networks. Polym. Chem. 2012, 3, 3121–3131. [Google Scholar] [CrossRef]
- Wenning, B.M.; Martinelli, E.; Mieszkin, S.; Finlay, J.A.; Fischer, D.; Callow, J.A.; Callow, M.E.; Leonardi, A.K.; Ober, C.K.; Galli, G. Model amphiphilic block copolymers with tailored molecular weight and composition in PDMS-based films to limit soft biofouling. ACS Appl. Mater. Interfaces 2017, 9, 16505–16516. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Wenning, B.; Rizis, G.; Calabrese, D.R.; Finlay, J.A.; Franco, S.C.; Zuckermann, R.N.; Clare, A.S.; Kramer, E.J.; Ober, C.K.; et al. Role of backbone chemistry and monomer sequence in amphiphilic oligopeptide- and oligopeptoid-functionalized PDMS- and PEO-based block copolymers for marine antifouling and fouling release coatings. Macromolecules 2017, 50, 2656–2667. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Gohad, N.V.; Eller, M.J.; Orihuela, B.; Rittschof, D.; Schweikert, E.A.; Mount, A.S.; Wooley, K.L. Noradrenaline-functionalized hyperbranched fluoropolymer-poly(ethylene glycol) cross-linked networks as dual-mode, anti-biofouling coatings. ACS Nano 2012, 6, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Kumar, S.; Mohanty, S.; Nayak, S.K. Environmentally benign fouling-resistant marine coatings: A review. Polym. Plast. Technol. Mater. 2019, 58, 498–518. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H.; Moacanin, J. A surface energy analysis of bioadhesion. Polymer 1977, 18, 475–482. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Update; Physical Electronics Division, Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- McIntyre, N.S.; Chan, T.C. Auger and X-ray Phototelectron Spectroscopy. In Practical Surface Analysis 1; Briggs, D., Seah, M.P., Eds.; Wiley: Chichester, UK, 1990; p. 485. [Google Scholar]









| Polymer | SzA/S a) | SzA (mol%) | SzA (wt %) | Mnb) (kg/mol) | Mnc) (kg/mol) | DPn c) SzA | Ðb) |
|---|---|---|---|---|---|---|---|
| S27SzA3 | 5 | 10 | 54 | 6.7 | 6.0 | 3 | 1.3 |
| S27SzA11 | 20 | 29 | 81 | 12.6 | 14.5 | 11 | 1.4 |
| S27SzA29 | 56 | 52 | 92 | 23.4 | 34.1 | 29 | 1.5 |
| Film | θa (°) a) | θr (°) a) | Δ (°) a) | θa (°) b) | θr (°) b) | Δ (°) b) |
|---|---|---|---|---|---|---|
| P(SzA) c) | 87 | 39 | 48 | 90 ± 0.8 | 38 ± 0.2 | 52 |
| S27SzA3 | 96 | 65 | 31 | 98 ± 0.4 | 64 ± 0.3 | 34 |
| S27SzA11 | 88 | 43 | 45 | 95 ± 0.6 | 41 ± 0.2 | 54 |
| S27SzA29 | 87 | 39 | 48 | 93 ± 0.5 | 38 ± 0.1 | 55 |
| Film | θw (°) | θh (°) |
|---|---|---|
| P(SzA) | 84 ± 1 | 62 ± 1 |
| S27SzA3 | 85 ± 1 | 64 ± 1 |
| S27SzA11 | 86 ± 3 | 65 ± 1 |
| S27SzA29 | 83 ± 1 | 65 ± 1 |
| Film | εmaxa) (%) | σmaxb) (MPa) | Ec) (MPa) |
|---|---|---|---|
| E30-S27SzA_0 | 513 ± 11 | 16.4 ± 0.8 | 3.6 ± 0.5 |
| E30-S27SzA3_100 | 510 ± 13 | 16.0 ± 1.1 | 3.4 ± 0.5 |
| E13- S27SzA_0 | 793 ± 51 | 10.2 ± 1.0 | 1.9 ± 0.0 |
| E13- S27SzA3_100 | 930 ± 25 | 13.1 ± 0.9 | 1.8 ± 0.0 |
| E13- S27SzA29_100 | 927 ± 52 | 12.3 ± 1.3 | 1.8 ± 0.0 |
| Film | θw (°) a) | θh (°) a) | γsOWK b) (mN/m) | γsd b) (mN/m) | γsp b) (mN/m) |
|---|---|---|---|---|---|
| E30-S27SzA_0 | 100 ± 1 | 28 ± 1 | 25.5 | 24.5 | 1.0 |
| E30-S27SzA11_100 | 99 ± 1 | 67 ± 1 | 17.0 | 13.4 | 3.6 |
| E30- S27SzA11_90 | 101 ± 1 | 65 ± 1 | 16.8 | 14.0 | 2.8 |
| E30-S27SzA29_100 | 104 ± 1 | 65 ± 1 | 16.0 | 14.0 | 2.0 |
| E30-S27SzA29_90 | 105 ± 1 | 64 ± 1 | 16.0 | 14.3 | 1.7 |
| E13-S27SzA_0 | 99 ± 1 | 39 ± 1 | 23.4 | 21.8 | 1.6 |
| E13-S27SzA3_100 | 96 ± 1 | 63 ± 2 | 18.9 | 14.6 | 4.3 |
| E13-S27SzA11_100 | 102 ± 1 | 64 ± 1 | 16.7 | 14.2 | 2.5 |
| E13-S27SzA11_90 | 101 ± 1 | 62 ± 1 | 17.5 | 14.9 | 2.6 |
| E13-S27SzA29_100 | 104 ± 1 | 63 ± 1 | 16.5 | 14.6 | 1.9 |
| E13-S27SzA29_90 | 105 ± 1 | 64 ± 1 | 16.0 | 14.3 | 1.7 |
| Film | φ (°) | Before Immersion | After Immersion | |||||
|---|---|---|---|---|---|---|---|---|
| C (%) | O (%) | F (%) | C (%) | O (%) | F (%) | |||
| S27SzA11 | Theor. a) | 67 | 15 | 18 | 67 | 15 | 18 | |
| 70 | 47.9 | 12.6 | 39.5 | 47.4 | 12.5 | 40.1 | ||
| E13-S27SzA11_100 | 50 | 52.8 | 13.6 | 33.6 | 52.1 | 13.7 | 34.2 | |
| 20 | 60.0 | 15.3 | 24.7 | 59.2 | 15.4 | 25.4 | ||
| S27SzA29 | Theor. a) | 62 | 17 | 21 | 62 | 17 | 21 | |
| 70 | 47.1 | 13.3 | 39.6 | 46.4 | 13.4 | 40.2 | ||
| E13-S27SzA29_100 | 50 | 50.8 | 15.1 | 34.1 | 50.1 | 15.6 | 34.3 | |
| 20 | 57.1 | 17.3 | 25.6 | 55.9 | 18.5 | 25.6 | ||
| 70 | 49.9 | 13.1 | 37.0 | 49.9 | 12.9 | 37.2 | ||
| E30-S27SzA11_90 | 50 | 53.4 | 14.7 | 31.9 | 53.4 | 13.6 | 33.0 | |
| 20 | 63.2 | 14.6 | 22.2 | 61.7 | 14.2 | 24.1 | ||
| 70 | 48.0 | 13.5 | 38.5 | 46.4 | 13.1 | 40.5 | ||
| E30-S27SzA29_90 | 50 | 51.0 | 15.1 | 33.9 | 50.5 | 14.6 | 34.9 | |
| 20 | 60.0 | 16.5 | 23.5 | 55.7 | 16.6 | 27.7 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guazzelli, E.; Galli, G.; Martinelli, E. The Effect of Poly(ethylene glycol) (PEG) Length on the Wettability and Surface Chemistry of PEG-Fluoroalkyl-Modified Polystyrene Diblock Copolymers and Their Two-Layer Films with Elastomer Matrix. Polymers 2020, 12, 1236. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061236
Guazzelli E, Galli G, Martinelli E. The Effect of Poly(ethylene glycol) (PEG) Length on the Wettability and Surface Chemistry of PEG-Fluoroalkyl-Modified Polystyrene Diblock Copolymers and Their Two-Layer Films with Elastomer Matrix. Polymers. 2020; 12(6):1236. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061236
Chicago/Turabian StyleGuazzelli, Elisa, Giancarlo Galli, and Elisa Martinelli. 2020. "The Effect of Poly(ethylene glycol) (PEG) Length on the Wettability and Surface Chemistry of PEG-Fluoroalkyl-Modified Polystyrene Diblock Copolymers and Their Two-Layer Films with Elastomer Matrix" Polymers 12, no. 6: 1236. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061236