Design and Synthesis of Free-Radical/Cationic Photosensitive Resin Applied for 3D Printer with Liquid Crystal Display (LCD) Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photosensitive Resins and 3D Printing Tests
2.3. Soxhlet Extraction Process
2.4. Characterization of the Samples
3. Results and Discussions
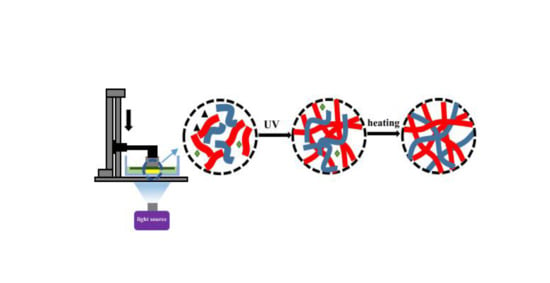
3.1. Mechanism of UV-Curing and LCD 3D Printing
3.2. FT-IR Analysis
3.3. Shrinkage of Photopolymer Resins
3.4. Tensile Strength and Elongation Properties
3.5. Effect of Heat Treatment at Different Temperatures on Spline Morphology
3.6. Soxhlet Extraction
3.7. Thermal Stability of the 3D-Printed Objects
3.8. Dynamic Mechanical Properties
3.9. Finite Element Analysis of the Effect of the Temperature on the Spline Warp
3.10. Microstructure of the Objects Printed by 3D Printer with LCD Light Source
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chattopadhyay, D.; Panda, S.S.; Raju, K. Thermal and mechanical properties of epoxy acrylate/methacrylates UV cured coatings. Prog. Org. Coat. 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Kardar, P.; Ebrahimi, M.; Bastani, S.; Jalili, M. Using mixture experimental design to study the effect of multifunctional acrylate monomers on UV cured epoxy acrylate resins. Prog. Org. Coat. 2009, 64, 74–80. [Google Scholar] [CrossRef]
- Buchanan, C.; Gardner, L. Metal 3D printing in construction: A review of methods, research, applications, opportunities and challenges. Eng. Struct. 2019, 180, 332–348. [Google Scholar] [CrossRef]
- He, Y.; Zhou, M.; Wu, B.; Jiang, Z.; Nie, J. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment. Prog. Org. Coat 2010, 67, 264–268. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Kahraman, M.V.; Kayaman-Apohan, A. The coating performance of adipic acid modified and methacrylated bisphenol-A based epoxy oligomers. Polym. Adv. Technol. 2007, 18, 173–179. [Google Scholar] [CrossRef]
- Hawker, J.; Hedrick, J.L.; Malmstrom, E.E. Dual living free radical and ring opening polymerizations from a double-headed initiator. Macromolecules. Macromolecules 1998, 31, 213–219. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S.; Nie, K. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Chiang, C.L.; Ma, C.C.M.; Wang, F.Y.; Kuan, H.C. Thermooxidative degradation of novel epoxy containing silicon and phosphorous nanocomposites. Eur. Polym. J. 2003, 39, 825–830. [Google Scholar] [CrossRef]
- Xu, F.; Yang, J.L.; Gong, Y.S.; Ma, G.P.; Nie, J. A fluorinated photoinitiator for surface oxygen inhibition resistance. Macromolecules 2012, 45, 1158–1164. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Arafa, A.A. Effect of process conditions on the properties of surface-modified organic pigments encapsulated by UV-curable resins. Coloration Technol. 2018, 134, 44–58. [Google Scholar] [CrossRef]
- Ceccia, S.; Turcato, E.A.; Maffettone, P.L.; Bongiovanni, R. Nanocomposite UV-curedcoatings: Organoclay intercalation by an epoxy resin. Prog. Org. Coat. 2008, 63, 110–115. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Turcato, E.A.; Gianni, A.D.; Ronchetti, S. Epoxy coatings containing clays and organoclays: Effect of the filler and its water content on the UV-curing process. Prog. Org. Coat. 2008, 62, 336–343. [Google Scholar] [CrossRef]
- Huang, B.; Du, Z. Preparation of a novel hybrid type photosensitive resin for stereolithography in 3D printing and testing on the accuracy of the fabricated parts. J. Wuhan Univ. Technol.-Mate 2017, 32, 726–732. [Google Scholar] [CrossRef]
- Sasaki, H. Curing properties of cycloaliphatic epoxy derivatives. Prog. Org. Coat. 2007, 58, 227–230. [Google Scholar] [CrossRef]
- Crivello, J.V. UV and electron beam-induced cationic polymerization. Nucl. Instrum. Methods Phys. Res. 1999, 151, 8–21. [Google Scholar] [CrossRef]
- Bulut, U.; Crivello, J.V. Investigation of the reactivity of epoxide monomers in photo-initiated cationic polymerization. Macromolecules 2005, 38, 3584–3595. [Google Scholar] [CrossRef]
- Teramoto, N.; Kogure, H.; Kimura, Y.; Shibata, M. Thermal properties and biodegradability of the copolymers of l-lactide, ε-caprolactone, and ethylene glycol oligomer with maleate units and their crosslinked products. Polymer 2004, 45, 7927–7933. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Lv, X.; Lu, H.; Hu, Y.; Yuen, R.K.K. Flame-retarded polystyrene with phosphorus- and nitrogen-containing oligomer: Preparation and thermal properties. J. Appl. Polym. 2011, 123, 770–778. [Google Scholar] [CrossRef]
- Kwak, R.; Park, H.; Ko, H. Partially Cured Photopolymer with Gradient Bingham Plastic Behaviors as a Versatile Deformable Material. ACS Macro Lett. 2017, 6, 561–565. [Google Scholar] [CrossRef]
- Ahn, B.U.; Lee, S.K.; Lee, S.K.; Park, J.H.; Kim, B.K. UV curable polyurethane dispersions from polyisocyanate and organosilane. Prog. Org. Coat. 2008, 62, 258–264. [Google Scholar] [CrossRef]
- Unal, S.; Oguz, C.; Yilgor, E.; Gallivan, M.; Long, T.E. Understanding the structure development in hyperbranched polymers prepared by oligomeric A2+B3 approach: Comparison of experimental results and simulations. Polymer 2005, 46, 4533–4543. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, R. A comparative study on 3D printed silicone-epoxy/acrylate hybrid polymers via pure photopolymerization and dual-curing mechanisms. J. Mater. Sci. 2019, 54, 5101–5111. [Google Scholar] [CrossRef]
- Colton, J.; Blair, B. Experimental study of post-build cure of stereolithography polymers for injection molds. Rapid Prototyp. J. 1999, 5, 72–81. [Google Scholar] [CrossRef]
- Hague, R.; Mansour, S.; Saleh, N. Materials analysis of stereolithography resins for use in Rapid Manufacturing. J. Mater. Sci. 2004, 39, 2457–2464. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, G.; Wang, S. Dynamic postpolymerization of 3D-printed photopolymer nanocomposites: Effect of cellulose nanocrystal and postcure temperature. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 935–946. [Google Scholar] [CrossRef]
- Green, W.A. Industrial Photoinitiators a Technical Guide; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Huang, J.; Yuan, T. UV/thermal dual curing of tung oil-based polymers induced by cationic photoinitiator. Prog. Org. Coat. 2019, 126, 8–17. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng 2012. [Google Scholar] [CrossRef] [Green Version]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Liu, F.J.; Liu, Y.P.; Liao, W.T. Hybrids of colloidal silica and waterborne polyurethane. J. Colloid Interface Sci. 2006, 302, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Van, G.A.; Fy, F. Theory of shrinkage stresses in oriented glass-reinforced plastics. Mechanika Polymerov. 1965, 6, 61–68. [Google Scholar]
- Karalekas, D.; Aggelopoulos, A. Study of shrinkage strains in a stereolithography cured acrylic photopolymer resin. J. Mater. Process. Technol. 2003, 136, 146–150. [Google Scholar] [CrossRef]
- Jin, H.; Yoon, S.; Kim, S.C. Synthesis and characterization of interpenetrating polymer networks from polyurethane and poly(ethylene glycol) diacrylate. J. Appl. Polym. 2008, 109, 805–812. [Google Scholar] [CrossRef]
- Okhawilai, M.; Pudhom, K.; Rimdusit, S. Synthesis and characterization of sequential interpenetrating polymer networks of polyurethane acrylate and polybenzoxazine. Polym. Eng. 2014, 54, 1151–1161. [Google Scholar] [CrossRef]
- Hossain, M.; Possart, G.; Steinmann, P. A small-strain model to simulate the curing of thermosets. Comput. Mech. 2009, 43, 769–779. [Google Scholar] [CrossRef]
- Wua, J.; Zhaoa, Z.; Hamel, C.M. Evolution of material properties during free radical photopolymerization. J. Mech. Phys. Solids 2018, 112, 25–49. [Google Scholar] [CrossRef]
- Hossain, M.; Possart, G.; Steinmann, P. A finite strain framework for the simulation of polymer curing. Part I: Elasticity. Comput. Mech. 2009, 44, 621–630. [Google Scholar] [CrossRef]













| Content (%) | Shrinkage (%) |
|---|---|
| 0 | 10.44 |
| 10 | 9.15 |
| 20 | 8.57 |
| 30 | 7.83 |
| 40 | 7.41 |
| Content /% | Tensile Strength/MPa | Breaking Elongation/% | Bending Strength/MPa |
|---|---|---|---|
| 0 | 25 | 17 | 47 |
| 10 | 29 | 12 | 58 |
| 20 | 28 | 14 | 49 |
| 30 | 22 | 21 | 44 |
| 40 | 13 | 30 | 27 |
| Sample | Gel Fraction (wt%) |
|---|---|
| Liquid resin | 0 |
| Freshly printed resin | 83.1 |
| 100 °C 1 h | 100 |
| 70 °C 2 h | 98.4 |
| 70 °C 3 h | 100 |
| Sample | Decomposition Temperature/°C | Residual Mass/% |
|---|---|---|
| 70 °C, 3 h | 395.22 | 15.74 |
| unheated | 380.22 | 14.31 |
| Sample | Tg (°C) | E′ (MPa) |
|---|---|---|
| A | 82.6 | 1826.9 |
| B | 79.8 | 1347.1 |
| C | 60.5 | 953.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, J.; Yang, Z.; Chen, G.; Hu, Y.; Luo, Y.; Dong, X.; Zheng, W.; Zhou, W. Design and Synthesis of Free-Radical/Cationic Photosensitive Resin Applied for 3D Printer with Liquid Crystal Display (LCD) Irradiation. Polymers 2020, 12, 1346. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061346
Shan J, Yang Z, Chen G, Hu Y, Luo Y, Dong X, Zheng W, Zhou W. Design and Synthesis of Free-Radical/Cationic Photosensitive Resin Applied for 3D Printer with Liquid Crystal Display (LCD) Irradiation. Polymers. 2020; 12(6):1346. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061346
Chicago/Turabian StyleShan, Junyang, Zijun Yang, Guoguang Chen, Yang Hu, Ying Luo, Xianming Dong, Wenxu Zheng, and Wuyi Zhou. 2020. "Design and Synthesis of Free-Radical/Cationic Photosensitive Resin Applied for 3D Printer with Liquid Crystal Display (LCD) Irradiation" Polymers 12, no. 6: 1346. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061346