Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films
Abstract
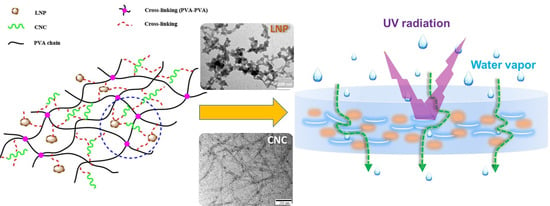
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA Nanocomposite Films
2.3. PVA Nanocomposite Film Characterization
2.3.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Wide-Angle X-Ray Diffraction (WAXD)
2.3.4. Tensile Properties
2.3.5. Microstructure
2.3.6. Optical Properties
2.3.7. Wettability
2.3.8. Overall Migration Activity
2.3.9. Antioxidant Activity
2.3.10. Water Vapour Transmission Rate (WVTR)
3. Results
3.1. FT-IR Analysis
3.2. Thermogravimetric Analysis (TGA)
3.3. WAXD
3.4. Tensile Properties
3.5. Microstructure
3.6. Wettability
3.7. Optical Properties
3.8. Overall Migration and Antioxidant Activity of Migrating Substances
3.9. WVTR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dotan, A. 15-Biobased Thermosets. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 577–622. [Google Scholar]
- Yang, W.; Fortunati, E.; Luzi, F.; Kenny, J.M.; Torre, L.; Puglia, D. Lignocellulosic Based Bionanocomposites for Different Industrial Applications. Curr. Org. Chem. 2018, 22, 1205–1221. [Google Scholar] [CrossRef]
- Sameni, J.; Jaffer, S.A.; Sain, M. Thermal and mechanical properties of soda lignin/HDPE blends. Compos. Part A Appl. Sci. Manuf. 2018, 115, 104–111. [Google Scholar] [CrossRef]
- Sapkota, J.; Natterodt, J.C.; Shirole, A.; Foster, E.J.; Weder, C. Fabrication and Properties of Polyethylene/Cellulose Nanocrystal Composites. Macromol. Mater. Eng. 2017, 302, 1600300. [Google Scholar] [CrossRef]
- Wu, B.; Zeng, Q.; Niu, D.; Yang, W.; Dong, W.; Chen, M.; Ma, P. Design of supertoughened and heat-resistant PLLA/elastomer blends by controlling the distribution of stereocomplex crystallites and the morphology. Macromolecules 2019, 52, 1092–1103. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Gao, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Yang, W.; Rallini, M.; Wang, D.-Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of lignin nanoparticles in UV resistance, thermal and mechanical performance of PMMA nanocomposites prepared by a combined free-radical graft polymerization/masterbatch procedure. Compos. Part A Appl. Sci. Manuf. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Dong, H.; Sliozberg, Y.R.; Snyder, J.F.; Steele, J.; Chantawansri, T.; Orlicki, J.A.; Walck, S.D.; Reiner, R.; Rudie, A.W. Highly Transparent and Toughened Poly(methyl methacrylate) Nanocomposite Films Containing Networks of Cellulose Nanofibrils. ACS Appl. Mater. Interfaces 2015, 7, 25464–25472. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Improved lignin polyurethane properties with lewis acid treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Fang, C.; Wang, S.; Huang, J.; Qiu, X. High-Performance Lignin-Containing Polyurethane Elastomers with Dynamic Covalent Polymer Networks. Macromolecules 2019, 52, 6474–6484. [Google Scholar] [CrossRef]
- Urbina, L.; Alonso-Varona, A.; Saralegi, A.; Palomares, T.; Eceiza, A.; Corcuera, M.Á.; Retegi, A. Hybrid and biocompatible cellulose/polyurethane nanocomposites with water-activated shape memory properties. Carbohydr. Polym. 2019, 216, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Rallini, M.; Natali, M.; Kenny, J.; Ma, P.; Dong, W.; Torre, L.; Puglia, D. Preparation and properties of adhesives based on phenolic resin containing lignin micro and nanoparticles: A comparative study. Mater. Des. 2019, 161, 55–63. [Google Scholar] [CrossRef]
- Cho, D.; Kim, J.M.; Kim, D. Phenolic resin infiltration and carbonization of cellulose-based bamboo fibers. Mater. Lett. 2013, 104, 24–27. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.-L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Sasaki, C.; Wanaka, M.; Takagi, H.; Tamura, S.; Asada, C.; Nakamura, Y. Evaluation of epoxy resins synthesized from steam-exploded bamboo lignin. Ind. Crop. Prod. 2013, 43, 757–761. [Google Scholar] [CrossRef]
- Nissilä, T.; Hietala, M.; Oksman, K. A method for preparing epoxy-cellulose nanofiber composites with an oriented structure. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105515. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly (vinyl alcohol)(PVA) and lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Liu, Y.; Bai, S.; Wang, Q. Thermal melt processing to prepare halogen-free flame retardant poly (vinyl alcohol). Polym. Degrad. Stab. 2014, 109, 261–269. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.-B.; Gregersen, Ø.W. Cellulose nanocrystal/PVA nanocomposite membranes for CO2/CH4 separation at high pressure. J. Membr. Sci. 2018, 554, 275–281. [Google Scholar] [CrossRef]
- Abral, H.; Hartono, A.; Hafizulhaq, F.; Handayani, D.; Sugiarti, E.; Pradipta, O. Characterization of PVA/cassava starch biocomposites fabricated with and without sonication using bacterial cellulose fiber loadings. Carbohydr. Polym. 2019, 206, 593–601. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Witt, M.A.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Mechanically improved polyvinyl alcohol-composite films using modified cellulose nanowhiskers as nano-reinforcement. Carbohydr. Polym. 2018, 191, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Oksman, K. Crosslinked poly(vinyl alcohol) composite films with cellulose nanocrystals: Mechanical and thermal properties. J. Appl. Polym. Sci. 2018, 135, 45710. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Honkaniemi, S.; Visanko, M.; Liimatainen, H. Composite Films of Poly(vinyl alcohol) and Bifunctional Cross-linking Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 19691–19699. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High Shear Homogenization of Lignin to Nanolignin and Thermal Stability of Nanolignin-Polyvinyl Alcohol Blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yang, D.; Qiu, X. Biomimetic Supertough and Strong Biodegradable Polymeric Materials with Improved Thermal Properties and Excellent UV-Blocking Performance. Adv. Funct. Mater. 2019, 29, 1806912. [Google Scholar] [CrossRef]
- Su, L.; Xing, Z.; Wang, D.; Xu, G.; Ren, S.; Fang, G. Mechanical properties research and structural characterization of alkali lignin/poly (vinyl alcohol) reaction films. BioResources 2013, 8, 3532–3543. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crop. Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly(lactic) acid films: Effect of cellulose nanocrystals and a masterbatch process. RSC Adv. 2015, 5, 32350–32357. [Google Scholar] [CrossRef]
- Yang, W.; Kenny, J.M.; Puglia, D. Structure and properties of biodegradable wheat gluten bionanocomposites containing lignin nanoparticles. Ind. Crop. Prod. 2015, 74, 348–356. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Environ. 2013, 21, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.-C. Structure and sorption properties of CNC reinforced PVA films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of polyvinyl alcohol (PVA) and effect of crosslinker shape (aliphatic and aromatic) thereof. J. Polym. Environ. 2018, 26, 1782–1794. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Luzi, F.; Santulli, C.; Kenny, J.M.; Torre, L. Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: Part I. Carbohydr. Polym. 2013, 97, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Hendrawan, H.; Khoerunnisa, F.; Sonjaya, Y.; Putri, A.D. Poly (vinyl alcohol)/glutaraldehyde/Premna oblongifolia merr extract hydrogel for controlled-release and water absorption application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Rudra, R.; Kumar, V.; Kundu, P.P. Acid catalysed cross-linking of poly vinyl alcohol (PVA) by glutaraldehyde: Effect of crosslink density on the characteristics of PVA membranes used in single chambered microbial fuel cells. RSC Adv. 2015, 5, 83436–83447. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Imtiyaz, K.; Rizvi, M.M.A.; Ahmad, S. Periodate-Modified Gum Arabic Cross-linked PVA Hydrogels: A Promising Approach toward Photoprotection and Sustained Delivery of Folic Acid. ACS Omega 2019, 4, 16026–16036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahaman, M.S.; Yeum, J.H. Electrospun novel super-absorbent based on polysaccharide–polyvinyl alcohol–montmorillonite clay nanocomposites. Carbohydr. Polym. 2015, 115, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Gholap, S.G.; Jog, J.P.; Badiger, M.V. Synthesis and characterization of hydrophobically modified poly (vinyl alcohol) hydrogel membrane. Polymer 2004, 45, 5863–5873. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qi, B.; Luo, J.; Wan, Y. Effect of alkali lignins with different molecular weights from alkali pretreated rice straw hydrolyzate on enzymatic hydrolysis. Bioresour. Technol. 2016, 200, 272–278. [Google Scholar] [CrossRef]
- de López Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of New Antioxidant Active Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Green Tea Extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef]
- Nerín, C.; Tovar, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008, 84, 313–320. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]










| Samples | Tp1 (°C) | Td1 (°C) | Td2 (°C) | Residual Mass (%) |
|---|---|---|---|---|
| PVA | 94.0 ± 0.2 | 323 ± 1.4 | - | 4.6 ± 0.1 |
| PVA-GD | 94.3 ± 0.1 | 318 ± 1.0 | 356 ± 1.4 | 4.8 ± 0.2 |
| P-2CNC | 93.8 ± 0.3 | 317 ± 0.8 | 364 ± 0.8 | 4.8 ± 0.1 |
| P-2CNC-1LNP | 97.0 ± 0.2 | 315 ± 0.5 | 365 ± 0.6 | 4.7 ± 0.2 |
| P-2CNC-2LNP | 97.2 ± 0.3 | 320 ± 1.2 | 363 ± 0.6 | 5.1 ± 0.1 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PVA | 26.0 ± 3.4 | 232 ± 50 |
| PVA-GD | 29.0 ± 2.2 | 170 ± 20 |
| P-2CNC | 31.6 ± 1.9 | 121 ± 20 |
| P-2CNC-1LNP | 35.4 ± 2.2 | 234 ± 30 |
| P-2CNC-2LNP | 35.2 ± 1.5 | 207 ± 35 |
| Samples | Absorbance (%) @517 nm | RSA (%) |
|---|---|---|
| PVA | 86.5 | 0 |
| PVA-GD | 77.2 | 10.7 |
| P-2CNC | 79.1 | 8.6 |
| P-2CNC-1LNP | 71.6 | 17.2 |
| P-2CNC-2LNP | 66.5 | 23.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers 2020, 12, 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061364
Yang W, Qi G, Kenny JM, Puglia D, Ma P. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers. 2020; 12(6):1364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061364
Chicago/Turabian StyleYang, Weijun, Guochuang Qi, José Maria Kenny, Debora Puglia, and Piming Ma. 2020. "Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films" Polymers 12, no. 6: 1364. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061364