Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Material for Extrusion-Based 3D Printing of Controlled-Release Tablet Shells
2.3. Process Optimization for 3D Printing of Controlled Release Tablet Shells
2.4. Design of 3D Printed Controlled-Release Tablet Shells
2.5. Evaluation of Propranolol HCl Tablet
2.5.1. Assay/Drug Content
2.5.2. In Vitro Dissolution Study
2.6. Encapsulation of Propranolol HCl Tablets
2.7. Characterization of Printed Controlled-Release Shell
2.7.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7.2. Differential Scanning Calorimetry (DSC)
2.8. In-Vitro Dissolution Study
2.8.1. Drug Release Profile
2.8.2. Drug Release Kinetics
3. Results and Discussion
3.1. Preparation of Material for 3D Printing of Controlled-Release Tablet Shells
3.2. Process Optimization for 3D Printing Controlled-Release Tablet Shells
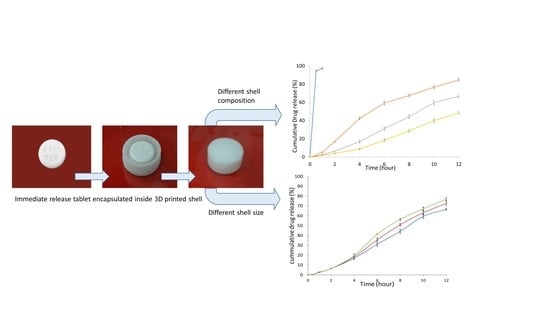
3.3. Design of 3D Printed Controlled-Release Tablet Shells
3.4. Printing of Controlled-Release Shells to Enclose Propranolol Hcl Tablets
3.5. Characterization of 3D Printed Controlled-Release Shells Enclosing the Immediate-Release Tablets
3.6. In Vitro Dissolution Study
3.6.1. Influence of the Composition of 3D Printed Controlled-Release Shells
3.6.2. Influence of the Size of the 3D Printed Controlled-Release Shell
3.6.3. Drug Release Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, J.U. Pharmacogenomics and personalized medicine. Nat. Educ. 2008, 1, 194. [Google Scholar]
- Tremblay, J.; Hamet, P. Role of genomics on the path to personalized medicine. Metabolism 2013, 62, S2–S5. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Soh, S.L. Printing Drug Tablets with Fully Customizable Release Profiles for Personalized Medicine. U.S. Patent Application No. 15/742, 754, 2 August 2018. [Google Scholar]
- Arafat, B.; Qinna, N.; Cieszynska, M.; Forbes, R.T.; Alhnan, M.A. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur. J. Pharm. Biopharm. 2018, 128, 282–289. [Google Scholar] [CrossRef]
- AlGahtani, M.S.; Mohammed, A.A.; Ahmad, J. Extrusion-based 3D printing for pharmaceuticals: Contemporary research and applications. Curr. Pharm. Des. 2019, 24, 4991–5008. [Google Scholar] [CrossRef]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control Release 2016, 238, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.M.; Sandler, N. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef] [Green Version]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.M.; Salonen, J.J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.; Donnelly, R.F.; Larrañeta, E. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef] [Green Version]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3d printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Gil, E.C.; Colarte, A.I.; Bataille, B.; Pedraz, J.L.; Rodríguez, F.; Heinämäki, J. Development and optimization of a novel sustained-release dextran tablet formulation for propranolol HCl. Int. J. Pharm. 2006, 317, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Kurcubic, I.; Cvijic, S.; Filipcev, B.; Ignjatovic, J.; Ibric, S.; Djuris, J. Development of propranolol HCl bilayer mucoadhesive buccal tablets supported by in silico physiologically-based modeling. React. Func. Polym. 2020, 151, 104587. [Google Scholar] [CrossRef]
- Medicines and Healthcare products Regulatory Agency. Propranolol HCl tablet. In British Pharmacopoeia; Medicines and Healthcare Products Regulatory Agency: London, UK, 2020; Volume III, pp. 1182–11833. [Google Scholar]
- United States Pharmacopoeial Convention. The United States Pharmacopeia 35; National Formulary 30; United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2012; Volume 3, p. 4465. [Google Scholar]
- Mehta, M.R.; Machhaliya, R.Y.; Patel, C.N.; Daraji, H.M. Formulation and evaluation of sublingual tablet of candesartan cilexetil. Int. J. Pharm. Res. BioSci. 2014, 3, 900–925. [Google Scholar]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Junior, J.M.M.; Muller, A.L.H.; Foletto, E.L.; da Costa, A.B.; Bizzi, C.; Müller, E.I. Determination of propranolol hydrochloride in pharmaceutical preparations using near infrared spectrometry with fiber optic probe and multivariate calibration methods. J. Anal. Methods Chem. 2015, 2015, 795102. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Iqbal, M.; Akhtar, N.; Khan, H.M.S.; Ullah, A.; Uddin, M.; Khan, M.T. Assessment of xanthan gum based sustained release matrix tablets containing highly water-soluble propranolol HCl. Acta Pol. Pharm. 2013, 70, 283–289. [Google Scholar] [PubMed]
- Sahoo, J.; Murthy, P.N.; Biswal, S.; Sahoo, S.K.; Mahapatra, A.K. Comparative study of propranolol hydrochloride release from matrix tablets with KollidonSR or hydroxy propyl methyl cellulose. AAPS PharmSciTech 2008, 9, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech 2011, 13, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Patel, M. Formulation and evaluation of controlled porosity osmotic drug delivery system of metoprolol succinate. Int. J. Pharm. Res. 2012, 3, 1761–1767. [Google Scholar]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [Green Version]
- Laboulfie, F.; Hémati, M.; Lamure, A.; Diguet, S. Effect of the plasticizer on permeability, mechanical resistance and thermal behaviour of composite coating films. Powder Technol. 2013, 238, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Brobyn, R.D. The human toxicology of dimethyl sulphoxide. Ann. N. Y. Acad. Sci. 1975, 243, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.W.; Jack, C. Dimethyl Sulfoxide (DMSO) in Trauma and Disease; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling of drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Kjar, A.; Huang, Y. Application of Micro-Scale 3D Printing in Pharmaceutics. Pharmaceutics 2019, 11, 390. [Google Scholar] [CrossRef] [Green Version]
- Grassi, M.; Grassi, G. Mathematical modelling and controlled drug delivery: Matrix systems. Curr. Drug Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A. Achieving zero-order release kinetics using multi-step diffusion-based drug delivery. Pharm. Tech. 2014, 26, 38–42. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]





| Solvent | Solubility | Solution Behavior and Extrudability Through the 3D Printer |
|---|---|---|
| Water | Insoluble | Insoluble mass |
| Water with surfactant (Tween 80) | Insoluble | Granular mass |
| Isopropyl alcohol | Insoluble | Insoluble mass |
| Ethanol | Insoluble | Insoluble mass |
| Dichloromethane | Insoluble | Insoluble mass |
| Ethyl acetate | Partially soluble | Segregated mass |
| Acetone | Soluble | Paste extruded rapidly, but tip blockage occurred due to drying of the paste over time |
| Acetone/water (1:1 ratio) | Insoluble | Segregated mass |
| Acetone/ethanol (1:1 ratio) | Insoluble | Gummy mass |
| Acetone/isopropyl alcohol (1:1 ratio) | Insoluble | Gummy mass |
| Acetone/propylene glycol (4:1 ratio) | Soluble | Paste but hindered extrusion |
| Acetone/isopropyl alcohol (3:2 ratio) | Soluble | Paste but no continuous extrusion |
| Acetone/ethanol/ dimethyl sulfoxide (DMSO) (2.5:2.5:1 ratio) | Soluble | Good uniformity and continuously extrudable paste |
| Process Parameters | Value | Outcome/Observation |
|---|---|---|
| Nozzle size (µm) | 200–400 µm | No extrusion |
| 400–600 µm | Good extrusion with an optimum layer thickness | |
| 600–800 µm | Very rapid extrusion with an increased layer thickness | |
| Extrusion pressure (psi) | 20–40 psi | No extrusion |
| 40–60 psi | Good extrusion with an optimum layer thickness | |
| 60–80 psi | Very rapid extrusion with an increased layer thickness | |
| Printing speed (mm/s) | 2 mm/s | Slow printing |
| 4 mm/s | Optimum printing | |
| 6 mm/s | Rapid printing, sagging due to wetting | |
| Nozzle shape | Blunt tip | Tip blockage and hindered extrusion |
| Tapered tip | Smooth and continuous extrusion |
| Formulation | % Composition Cellulose Acetate D–Mannitol PEG 6000 | % Cumulative Drug Release After 12 h | ||
|---|---|---|---|---|
| Shell A | 20 | 65 | 15 | 84.64 ± 1.99 |
| Shell B | 40 | 45 | 15 | 66.60 ± 1.13 |
| Shell C | 60 | 25 | 15 | 48.37 ± 1.46 |
| Shell Size | Shell Dimensions | % Cumulative Drug Release After 12 h | ||
|---|---|---|---|---|
| Shell Outer Diameter | Shell Inner Diameter | The Gap between the Shell Wall and Tablet | ||
| Fixed-sized shell | 12 mm | 9.6 mm | 0.8 mm | 66.60 ± 1.13 |
| Medium-sized shell | 13 mm | 10.6 mm | 1.3 mm | 72.68 ± 2.34 |
| Large-sized shell | 14 mm | 11.6 mm | 1.8 mm | 76.81 ± 1.60 |
| Formulation Type | Zero Order (r2) | First Order (r2) | Higuchi (r2) | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| (r2) | n Value | ||||
| Composition-based | |||||
| Formulation A | 0.9521 | 0.9939 | 0.9906 | 0.9662 | 0.542 |
| Formulation B | 0.9937 | 0.9741 | 0.9503 | 0.9954 | 0.619 |
| Formulation C | 0.9817 | 0.9541 | 0.9106 | 0.9959 | 0.576 |
| Size-based | |||||
| Fixed-sized shell | 0.9937 | 0.9741 | 0.9503 | 0.9954 | 0.619 |
| Medium-sized shell | 0.9949 | 0.9622 | 0.9516 | 0.9964 | 0.621 |
| Large-sized shell | 0.9902 | 0.9583 | 0.9583 | 0.9942 | 0.622 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers 2020, 12, 1395. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061395
Algahtani MS, Mohammed AA, Ahmad J, Saleh E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers. 2020; 12(6):1395. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061395
Chicago/Turabian StyleAlgahtani, Mohammed S., Abdul Aleem Mohammed, Javed Ahmad, and Ehab Saleh. 2020. "Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets" Polymers 12, no. 6: 1395. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12061395