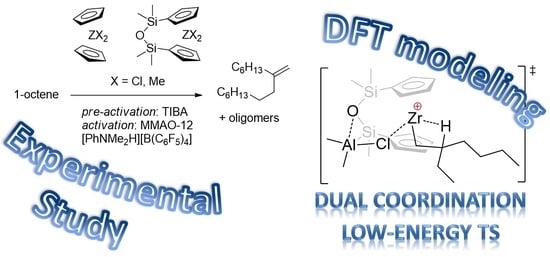
Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimentsl Remarks
2.2. Synthesis of Zirconocene 2′
2.3. Oligomerization Experiments
2.4. DFT Calculations
3. Results and Discussion
3.1. Oligomerization Experiments and End-Group Analysis
3.2. DFT Modeling of the Reaction Pathways for (η5-C5H5)2Zr-Based Catalytic Species
3.2.1. Mononuclear Reaction Mechanism
3.2.2. The Effect of the Formation of Zr-Al1 Species on the Reaction Pathway
3.2.3. Theoretical Analysis of the Possible Participation of Zr-Al2 Species
3.3. DFT Modeling of the Reaction Pathways for O[SiMe2(η5-C5H4)]2Zr-Based Catalytic Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Mohring, P.C.; Coville, N.J. Group 4 metallocene polymerisation catalysts: Quantification of ring substituent steric effects. Coord. Chem. Rev. 2006, 250, 18–35. [Google Scholar] [CrossRef]
- Chirik, P.J. Group 4 transition metal sandwich complexes: Still fresh after almost 60 years. Organometallics 2010, 29, 1500–1517. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D. Development of ansa-metallocene catalysts for isotactic olefin polymerization. In Polyolefins: 50 Years after Ziegler and Natta; Kaminsky, W., Ed.; Book Series Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 258, pp. 29–42. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Single site metallorganic polymerization catalysis as a method to probe the properties of polyolefins. Polym. Chem. 2011, 2, 2155–2168. [Google Scholar] [CrossRef]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 metal complexes for homogeneous olefin polymerisation: A short tutorial review. Appl. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Desert, X.; Carpentier, J.-F.; Kirillov, E. Quantification of active sites in single-site group 4 metal olefin polymerization catalysis. Coord. Chem. Rev. 2019, 386, 50–68. [Google Scholar] [CrossRef]
- Castro, L.; Kirillov, E.; Miserque, O.; Welle, A.; Haspeslagh, L.; Carpentier, J.-F.; Maron, L. Are solvent and dispersion effects crucial in olefin polymerization DFT calculations? Some insights from propylene coordination and insertion reactions with group 3 and 4 metallocenes. ACS Catal. 2015, 5, 416–425. [Google Scholar] [CrossRef]
- Desert, X.; Proutiere, F.; Welle, A.; Den Dauw, K.; Vantomme, A.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Kirillov, E. Zirconocene-catalyzed polymerization of α-olefins: When intrinsic higher activity is flawed by rapid deactivation. Organometallics 2019, 38, 2664–2673. [Google Scholar] [CrossRef]
- Kissin, Y.V. Oligomerization reactions of 1-hexene with metallocene catalysts: Detailed data on reaction chemistry and kinetics. Mol. Catal. 2019, 463, 87–93. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Explaining some anomalies in catalytic activity values in some zirconocene methyl cations: Local hyper-softness. J. Phys. Chem. C 2013, 117, 24773–24786. [Google Scholar] [CrossRef]
- Bravo, I.; Alonso-Moreno, C.; Carrillo-Hermosilla, F.; López-Solera, I.; Antiñolo, A.; Albaladejo, J. Toward the prediction of activity in the ethylene polymerisation of ansa-Bis(indenyl) zirconocenes: Effect of the stereochemistry and hydrogenation of the indenyl moiety. ChemPlusChem 2015, 80, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Cavallo, L.; Talarico, G. Buried volume analysis for propene polymerization catalysis promoted by group 4 metals: A tool for molecular mass prediction. ACS Catal. 2015, 5, 6815–6822. [Google Scholar] [CrossRef] [Green Version]
- Zaccaria, F.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V.; Ehm, C. Backbone rearrangement during olefin capture as the rate limiting step in molecular olefin polymerization catalysis and its effect on comonomer affinity. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2807–2814. [Google Scholar] [CrossRef]
- Zaccaria, F.; Ehm, C.; Budzelaar, P.H.M.; Busico, V. Accurate prediction of copolymerization statistics in molecular olefin polymerization catalysis: The role of entropic, electronic, and steric effects in catalyst comonomer affinity. ACS Catal. 2017, 7, 1512–1519. [Google Scholar] [CrossRef]
- Parveen, R.; Cundari, T.R.; Younker, J.M.; Rodriguez, G.; McCullough, L. DFT and QSAR studies of ethylene polymerization by zirconocene catalysts. ACS Catal. 2019, 9, 9339–9349. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Arlman, E.J. Ziegler-Natta catalysis II. Surface structure of layer-lattice transition metal chlorides. J. Catal. 1964, 3, 89–98. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3-AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Borrelli, M.; Busico, V.; Cipullo, R.; Ronca, S.; Budzelaar, P.H.M. Selectivity of metallocene-catalyzed olefin polymerization: A combined experimental and quantum mechanical study. 1. Nonchiral bis(cyclopentadienyl) systems. Macromolecules 2002, 35, 2835–2844. [Google Scholar] [CrossRef]
- Moscardi, G.; Resconi, L.; Cavallo, L. Propene polymerization with the isospecific, highly regioselective rac-Me2C(3-t-Bu-1-Ind)2ZrCl2/MAO catalyst. 2. Combined DFT/MM analysis of chain propagation and chain release reactions. Organometallics 2001, 20, 1918–1931. [Google Scholar] [CrossRef]
- Silanes, I.; Ugalde, J.M. Comparative study of various mechanisms for metallocene-catalyzed α-olefin polymerization. Organometallics 2005, 24, 3233–3246. [Google Scholar] [CrossRef]
- Chan, M.S.W.; Vanka, K.; Pye, C.C.; Ziegler, T. Density functional study on activation and ion-pair formation in group IV metallocene and related olefin polymerization catalysts. Organometallics 1999, 18, 4624–4636. [Google Scholar] [CrossRef]
- Woo, T.K.; Fan, L.; Ziegler, T. A density functional study of chain growing and chain terminating steps in olefin polymerization by metallocene and constrained geometry catalysts. Organometallics 1994, 13, 2252–2261. [Google Scholar] [CrossRef]
- Silanes, I.; Mercero, J.M.; Ugalde, J.M. Comparison of Ti, Zr, and Hf as cations for metallocene-catalyzed olefin polymerization. Organometallics 2006, 25, 4483–4490. [Google Scholar] [CrossRef]
- Laine, A.; Linnolahti, M.; Pakkanen, T.A.; Severn, J.R.; Kokko, E.; Pakkanen, A. Comparative theoretical study on homopolymerization of α-olefins by bis(cyclopentadienyl) zirconocene and hafnocene: Elemental propagation and termination reactions between monomers and metals. Organometallics 2010, 29, 1541–1550. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4658078, 14 April 1987. [Google Scholar]
- Christoffers, J.; Bergman, R.G. Catalytic dimerization reactions of α-olefins and α,ω-dienes with Cp2ZrCl2/poly(methylalumoxane): Formation of dimers, carbocycles, and oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1)—A catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Takeuchi, K.; Fujikawa, S. Base Oil for Oil Drilling Fluid and Oil Drilling Fluid Composition. U.S. Patent 2011251445, 13 October 2011. [Google Scholar]
- Kissin, Y.V.; Schwab, F.C. Post-oligomerization of alpha-olefin oligomers: A route to single-component and multicomponent synthetic lubricating oils. J. Appl. Polym. Sci. 2009, 111, 273–280. [Google Scholar] [CrossRef]
- Heilman, W.J.; Jois, Y.H.; De Kraker, A.R.; Song, W. Hydrocarbon Compositions Useful as Lubricants. U.S. Patent 2011178348, 1 May 2011. [Google Scholar]
- Fujikawa, S.; Yokota, K.; Okano, M.; Tsuji, M. Method for producing α-olefin oligomers and lubricating oil compositions. U.S. Patent Application 2011207977, 25 August 2011. [Google Scholar]
- Janiak, C.; Blank, F. Metallocene catalysts for olefin oligomerization. Macromol. Symp. 2006, 236, 14–22. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene ‘dimer’. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Fujikawa, S.; Okamoto, T.; Yokota, K. Process for Producing Unsaturated Hydrocarbon Compound. U.S. Patent 8119850, 21 February 2012. [Google Scholar]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Kissin, Y.V. Detailed kinetics of 1-hexene oligomerization reaction with (n-Bu-Cp)2ZrCl2–MAO catalyst. Macromol. Chem. Phys. 2009, 210, 1241–1246. [Google Scholar] [CrossRef]
- Graeper, J.; Paolucci, G.; Fischer, R.D. Zirconocenophane dichlorides with di- and trisiloxane-bridged ring ligands: Crystal structure of rac-[1,1,3,3-tetramethyldisiloxane-diyl-bis(3-tert-butyl-η5-cyclopentadienyl)zirconium(IV) dichloride. J. Organomet. Chem. 1995, 501, 211–218. [Google Scholar] [CrossRef]
- Samuel, E.; Rausch, M.D. π-Cyclopentadienyl and π-indenyl compounds of titanium, zirconium, and hafnium containing σ-bonded organic substituents. J. Am. Chem. Soc. 1973, 95, 6263–6267. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other fun. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part, I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H. Performance of density functionals for activation energies of Zr-mediated reactions. J. Chem. Theory Comput. 2013, 9, 4735–4743. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The catalytic behavior of heterocenes activated by TIBA and MMAO under a low Al/Zr ratios in 1-octene polymerization. Appl. Catal. A Gen. 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Kawahara, N.; Saito, J.; Matsuo, S.; Kaneko, H.; Matsugi, T.; Toda, Y.; Kashiwa, N. Study on unsaturated structures of polyhexene, poly(4-methylpentene) and poly(3-methylpentene) prepared with metallocene catalysts. Polymer 2007, 48, 425–428. [Google Scholar] [CrossRef]
- Kim, I.; Zhou, J.-M.; Chung, H. Higher α-olefin polymerizations catalyzed by rac-Me2Si(1-C5H2-2-CH3-4-tBu)2Zr(NMe2)2/Al(iBu)3/[Ph3C][B(C6F5)4]. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1687–1697. [Google Scholar] [CrossRef]
- Babu, G.N.; Newmark, R.A.; Chien, J.C.W. Microstructure of poly(1-hexene) produced by ansa-zirconocenium catalysis. Macromolecules 1994, 27, 3383–3388. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of higher 1-olefins with metallocene/MAO catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.H. Zirconium allyl complexes as participants in zirconocene-catalyzed alpha-olefin polymerizations. Angew. Chem. Int. Ed. 2014, 53, 9645–9649. [Google Scholar] [CrossRef]
- Brant, P.; Jiang, P.; Lovell, J.; Crowther, D. Termination events in sterically hindered metallocene-catalyzed olefin oligomerizations: Vinyl chain ends in oligooctenes. Organometallics 2016, 35, 2836–2839. [Google Scholar] [CrossRef]
- Wu, M.M.; Pafford, B.J.; Stavens, K.B. Polyalphaolefins by Oligomerization and Isomerization. U.S. Patent application 2014323665, 30 October 2014. [Google Scholar]
- Park, J.H.; Jang, Y.E.; Jeon, J.Y.; Go, M.J.; Lee, J.; Kim, S.K.; Lee, S.-I.; Lee, B.Y. Preparation of ansa-metallocenes for production of poly(α-olefin) lubricants. Dalton Trans. 2014, 43, 10132–10138. [Google Scholar] [CrossRef]
- Wu, M.M.; Hagemeister, M.P.; Yang, N. Process to Produce Polyalphaolefins. U.S. Patent 8513478, 20 August 2013. [Google Scholar]
- Welle, A.; Wassenaar, J.; Slawinski, M. Use of a Metallocene Catalyst to Produce a Polyalpha-Olefin. U.S. Patent 9688792, 27 June 2017. [Google Scholar]
- Shimizu, H.; Katayama, K.; Noda, H.; Okano, M. 1-Octene, 1-decene, 1-dodecene ternary copolymer and lubricants therewith. U.S. Patent 2014256997, 11 September 2014. [Google Scholar]
- Patil, A.O.; Bodige, S. Synthetic Libricant Basestocks and Methods of Preparation Thereof. U.S. Patent 2014046878, 23 August 2016. [Google Scholar]
- Patil, A.O.; Bodige, S.; Luo, S.; Chu, J.W.; Stavens, K.; Harrrington, B.A. Ultra High Viscosity Synthetic Base Stocks and Process for Preparing Same. U.S. Patent 2014213834, 31 July 2014. [Google Scholar]
- Wu, M.M.; Rucker, S.P.; Canich, A.M. Process for Producing Novel Synthetic Basestocks. U.S. Patent 9701595, 11 July 2017. [Google Scholar]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-catalyzed dimerization of α-olefins: DFT modeling of the Zr-Al binuclear reaction mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiak, C.; Lange, K.C.H.; Marquardt, P. Alkyl-substituted cyclopentadienyl- and phospholyl-zirconium/MAO catalysts for propene and 1-hexene oligomerization. J. Mol. Catal. A Chem. 2002, 180, 43–58. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P.; Krüger, R.-P.; Hanselmann, R. Analyses of propene and 1-hexene oligomers from zirconocene/MAO catalysts—Mechanistic implications by NMR, SEC, and MALDI-TOF MS. Macromol. Chem. Phys. 2002, 203, 129–138. [Google Scholar] [CrossRef]
- Marks, T.J.; Yang, X. Homogeneous Alpha-Olefin Dimerization Catalysts. U.S. Patent 5500398, 19 March 1996. [Google Scholar]
- Margl, P.M.; Woo, T.K.; Ziegler, T. Potential catalyst deactivation Reaction in homogeneous Ziegler−Natta polymerization of olefins: Formation of an allyl intermediate. Organometallics 1998, 17, 4997–5002. [Google Scholar] [CrossRef]
- Lieber, S.; Prosenc, M.-H.; Brintzinger, H.-H. Zirconocene allyl complexes: Dynamics in solution, reaction with aluminum alkyls, B(C6F5)3-induced propene insertion, and density-functional calculations on possible formation and reaction pathways. Organometallics 2000, 19, 377–387. [Google Scholar] [CrossRef]
- Landis, C.R.; Christianson, M.D. Metallocene-catalyzed alkene polymerization and the observation of Zr-allyls. Proc. Natl. Acad. Sci. USA 2006, 103, 15349–15354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatamanu, M. Synthesis, structures, and dynamic features of d0 zirconocene–allyl complexes. Organometallics 2014, 33, 3683–3694. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.-H. Zirconium-allyl complexes as resting states in zirconocene-catalyzed a-olefin polymerization. Macromol. Rapid Commun. 2015, 36, 249–253. [Google Scholar] [CrossRef]
- Vatamanu, M. Observation of zirconium allyl species formed during zirconocene-catalyzed propene polymerization and mechanistic insights. J. Catal. 2015, 323, 112–120. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Babushkin, D.E.; Bercaw, J.E.; Brintzinger, H.H. Catalyst speciation during ansa-zirconocene-catalyzed polymerization of 1-hexene studied by UV-vis spectroscopy—Formation and partial re-activation of Zr-Allyl intermediates. Polymers 2019, 11, 936. [Google Scholar] [CrossRef] [Green Version]
- Götz, C.; Rau, A.; Luft, G. Ternary metallocene catalyst systems based on metallocene dichlorides and AlBu3i/[PhNMe2H][B(C6F5)4]: NMR investigations of the influence of Al/Zr ratios on alkylation and on formation of the precursor of the active metallocene species. J. Mol. Catal. A Chem. 2002, 174, 95–110. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2···ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Sizov, A.I.; Zvukova, T.M.; Belsky, V.K.; Bulychev, B.M. Aluminium zirconium (+3 and +4) heterometallic hydrido complexes of compositions [(η5-C5H5)2Zr(μ-H)]2(μ-H)AlCl2 and [(η5-C5H5)2ZrH(μ-H)2]3Al. J. Organomet. Chem. 2001, 619, 36–42. [Google Scholar] [CrossRef]
- Wehmschulte, R.J.; Power, P.P. Reaction of cyclopentadienyl zirconium derivatives with sterically encumbered arylaluminum hydrides: X-ray crystal structure of (η5-C5H5)2Zr(μ2-H)2AlC6H2-2,4,6-But3. Polyhedron 1999, 18, 1885–1888. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zakharov, V.A.; Brintzinger, H.H. Novel zirconocene hydride complexes in homogeneous and in SiO2-supported olefin-polymerization catalysts modified with diisobutylaluminium hydride or triisobutylaluminum. Macromol. Chem. Phys. 2008, 209, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanisms of reactions of organoaluminium compounds with alkenes and alkynes catalyzed by Zr complexes. Russ. Chem. Rev. 2012, 81, 524–548. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Balaev, A.V.; Gubaidullin, I.M.; Abzalilova, L.R.; Pechatkina, S.V.; Khalilov, L.M.; Spivak, S.I.; Dzhemilev, U.M. Kinetic model of olefin hydroalumination by HAlBui2 and AlBui3 in the presence of Cp2ZrCl2 catalyst. Int. J. Chem. Kinet. 2007, 39, 333–339. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al hydride intermediate structure and dynamics in alkene hydroalumination with XAlBui2 (X = H, Cl, Bui), catalyzed by Zr η5 complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 Catalyst. I. Simulation of intermediate formation in reaction of HAlBui2 with Cp2ZrCl2. Organometallics 2009, 28, 968–977. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT and Ab initio study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 catalyst. II. Olefin interaction with catalytically active centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-complexed zirconocene hydrides: Identification of hydride-bridged species by NMR spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr, Al-hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Van der Heijden, H.; Hessen, B.; Orpen, A.G. A zwitterionic zirconocene alkyl complex as a single-component α-olefin dimerization catalyst. J. Am. Chem. Soc. 1998, 120, 1112–1113. [Google Scholar] [CrossRef] [Green Version]
- Bochmann, M.; Lancaster, S.J. Monomer–dimer equilibria in homo- and heterodinuclear cationic alkylzirconium complexes and their role in polymerization catalysis. Angew. Chem. Int. Ed. 1994, 33, 1634–1637. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.-H. Activation of dimethyl zirconocene by methylaluminoxane (MAO)-size estimate for Me-MAO– anions by pulsed field-gradient NMR. J. Am. Chem. Soc. 2002, 124, 12869–12873. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bader, M.; Marquet, N.; Bondon, A.; Roisnel, T.; Guegan, J.-P.; Amar, A.; Boucekkine, A.; Carpentier, J.-F.; Kirillov, E. Discrete ionic complexes of highly isoselective zirconocenes. solution dynamics, trimethylaluminum adducts, and implications in propylene polymerization. Organometallics 2016, 35, 258–276. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bondon, A.; Dorcet, V.; Carpentier, J.-F.; Kirillov, E. Heterobi- and -trimetallic ion pairs of zirconocene-based isoselective olefin polymerization catalysts with AlMe3. Angew. Chem. Int. Ed. 2015, 54, 6343–6346. [Google Scholar] [CrossRef]
- Ehm, C.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V. Role of TMA in polymerization. Dalton Trans. 2016, 45, 6847–6855. [Google Scholar] [CrossRef]
- Guo, Y.; He, F.; Zhang, Z.; Khan, A.; Fu, Z.; Xu, J.; Fan, Z. Influence of trimethylaluminum on kinetics of rac-Et(Ind)ZrCl2/aluminoxane catalyzed ethylene polymerization. J. Organomet. Chem. 2016, 808, 109–116. [Google Scholar] [CrossRef]
- Collins, S.; Linnolahti, M.; Garcia Zamora, M.; Zijlstra, H.S.; Rodríguez Hernández, M.T.; Perez-Camacho, O. Activation of Cp2ZrX2 (X = Me, Cl) by methylaluminoxane as studied by electrospray ionization mass spectrometry: Relationship to polymerization catalysis. Macromolecules 2017, 50, 8871–8884. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic alkylaluminum-complexed zirconocene hydrides as participants in olefin polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuklin, M.S.; Hirvi, J.T.; Bochmann, M.; Linnolahti, M. Toward controlling the metallocene/methylaluminoxane-catalyzed olefin polymerization process by a computational approach. Organometallics 2015, 34, 3586–3597. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Henling, L.M.; Day, M.W.; Brintzinger, H.H. Cationic alkylaluminum-complexed zirconocene hydrides: NMR-spectroscopic identification, crystallographic structure determination, and interconversion with other zirconocene cations. J. Am. Chem. Soc. 2011, 133, 1805–1813. [Google Scholar] [CrossRef] [Green Version]












| Run | Pre- cat. | TiBA/Zr Ratio | Activator | [Act]/ [Precat] Ratio | H2 | Conv. % | Dimer (C16), Trimer (C24), Tetramer (C32) and Pentamer (C40) wt. % in the Products | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C16 | C24 | C32 | C40 | |||||||
| 1 | 1 | 20 | MMAO-12 | 10 | – | 85 | 88.8 | 9.2 | 2.1 | – |
| 2 | 1 | 20 | MMAO-12 +1 eq. Et2AlCl | 10 | – | 74 | 90.3 | 7.8 | 1.9 | – |
| 3 | 1 | 20 | MMAO-12 | 10 | 1 bar | 84 | 92.0 | 6.7 | 1.3 | – |
| 4 | 1 | 20 | MMAO-12 | 200 | – | 68 | 77.6 | 15.1 | 5.4 | 1.9 |
| 5 | 1 | 20 | NBF | 1 | – | 53 | 81.6 | 13.2 | 4.2 | 1.0 |
| 6 | 1 | 20 | NBF | 1 | 1 bar | 70 | 84.6 | 11.6 | 3.3 | 0.5 |
| 7 | 1′ | – | MMAO-12 | 10 | – | 47 | 88.7 | 8.6 | 2.6 | – |
| 8 | 1′ | – | MMAO-12 +1 eq. Et2AlCl | 10 | – | 60 | 93.4 | 5.6 | 1.0 | – |
| 9 | 1′ | – | NBF | 1 | – | 14 | 78.2 | 17.7 | 4.0 | – |
| 10 | 1′ | 20 | NBF | 1 | – | 8 | 75.2 | 18.9 | 5.8 | – |
| 11 | 1′ | – | NBF +1 eq. Et2AlCl | 1 | – | 7 | 90.7 | 8.2 | 1.0 | – |
| 12 | 1′ | 20 | NBF | 1 | 1 bar | 18 | 72.3 | 16.8 | 9.2 | 1.7 |
| 13 | 2 | 20 | MMAO-12 | 10 | – | 82 | 92.4 | 7.1 | 0.5 | – |
| 14 | 2 | 20 | MMAO-12 +1 eq. Et2AlCl | 10 | – | 79 | 96.3 | 3.5 | 0.2 | – |
| 15 | 2 | 20 | MMAO-12 | 10 | 1 bar | 86 | 92.3 | 7.2 | 0.5 | – |
| 16 | 2 | 20 | MMAO-12 | 200 | – | 70 | 78.1 | 15.8 | 5.7 | 0.4 |
| 17 | 2 | 20 | NBF | 1 | – | 68 | 67.7 | 23.3 | 7.6 | 1.4 |
| 18 | 2 | 20 | NBF | 1 | 1 bar | 85 | 82.2 | 13.6 | 3.4 | 0.8 |
| 19 | 2′ | – | MMAO-12 | 10 | – | 65 | 58.4 | 25.1 | 11.5 | 5.0 |
| 20 | 2′ | – | MMAO-12 +1 eq. Et2AlCl | 10 | – | 57 | 87.6 | 11.2 | 1.2 | – |
| 21 | 2′ | – | NBF | 1 | – | 60 | 25.2 | 25.2 | 22.9 | 26.7 |
| 22 | 2′ | 20 | NBF | 1 | – | 13 | 47.4 | 21.7 | 15.6 | 15.2 |
| 23 | 2′ | – | NBF +1 eq. Et2AlCl | 1 | – | 38 | 70.3 | 22.1 | 6.3 | 1.3 |
| 24 | 2′ | – | NBF | 1 | 1 bar | 73 | 48.3 | 26.0 | 14.6 | 11.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12071590
Nifant’ev I, Vinogradov A, Vinogradov A, Karchevsky S, Ivchenko P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers. 2020; 12(7):1590. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12071590
Chicago/Turabian StyleNifant’ev, Ilya, Alexander Vinogradov, Alexey Vinogradov, Stanislav Karchevsky, and Pavel Ivchenko. 2020. "Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene" Polymers 12, no. 7: 1590. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12071590