Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
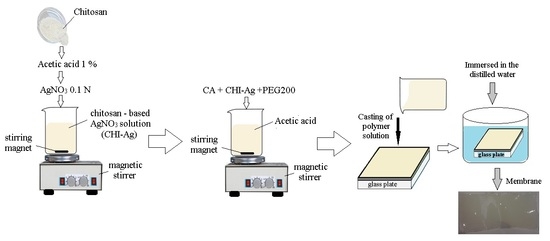
2.2. Biopolymeric Membrane Preparation
2.3. Characterization Techniques and Instrumentation
2.3.1. Fourier Transforms Infrared Spectroscopy-Attenuated Total Reflection (FTIR-ATR)
2.3.2. Contact Angle and Surface Free Energy (SFE)
2.3.3. Water Uptake
2.3.4. Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DSC)
2.3.5. Microscopy Studies
2.3.6. Electrochemical Impedance Spectroscopy
2.4. Electrodialysis Equipment and Procedure
3. Results and Discussion
3.1. Membranes Characterization
3.1.1. FTIR-ATR Spectroscopy Characterization of Membranes
- The stretching vibration band related to the—OH group (νO-H) was observed at 3405 cm−1 for the polymeric membrane without chitosan-silver ions [44]. An intense absorption band was observed at 3395 cm−1 when CHI-Ag was added to polymer mixture, corresponding to νO-H overlap to the stretching vibration of N–H (νN-H) from the free amino group (-NH2) at the C2 position of glucosamine from CHI. This absorption band registered by prominent peaks suggests the hydrogen bonds formed between components. Comparing the membranes’ spectra, it can be observed that the presence of CHI-Ag into the composition led to a more intense peak shifted to lower wavenumber (about ~10 cm−1).
- The aliphatic C-H stretching was registered at 2956 cm−1 and 2897 cm−1, respectively, in the polymeric membrane without chitosan-silver ions (CA/PEG/CHI-Ag membrane) [45].
- The same band corresponding to valence’s vibrations of C-H (νasC–H) in –CH2– and in –CH3 (νs C–H) appeared at 2956 cm−1, 2920 cm−1, and 2853 cm−1 in the case of the polymeric membrane enriched with chitosan-silver ions. The new peak at 2920 cm−1 and the shifting from 2897 cm−1 to 2853 cm−1 are due to the CHI-Ag incorporation in the mixture.
- Vibrations of–OH groups from PEG and CHI are evidenced by the peak at around 1431 cm−1 corresponding to δCH2 in CH2OH groups.
- The peaks at around 1370 cm−1 and 1320 cm−1, respectively, appeared in both membranes and are related to the vibrations of –OH and –CH groups in the pyranose ring [48].
- Between 1159 cm−1 and 1035 cm−1, the presence of distinct vibrational modes like –C–O–C (νC-O-C in glycosidic linkage) and C-OH (νC-O in primary OH group) are noticed.
3.1.2. Contact Angle and Surface Free Energy (SFE) Determination
3.1.3. Water Uptake
3.1.4. Thermal Gravimetric Analysis (TGA) and Differential Thermal Analysis (DSC) Characterization
3.1.5. Microscopic Analysis
3.1.6. Electrochemical Impedance Spectroscopy Tests
3.2. Performance Evaluation of Electrodialysis Cell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaturvedi, S.; Dave, P.N. Removal of iron for safe drinking water. Desalination 2012, 303, 1–11. [Google Scholar] [CrossRef]
- Marchovecchio, R.H.; Botte, J.E.; Freiji, S.E. Heavy metals, major metals, trace elements. In Handbook Water Analysis; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ngah, W.S.W.; Ghani, S.A.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2005, 96, 443–450. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for drinking-water quality. In Recomendations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008; Volume 1, pp. 390–399. [Google Scholar]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Constantin, L.; Constantin, A.M.; Cărăuşu, M.E.; Isildak, I. Added value recyclability of glass fiber waste as photo-oxidation catalyst for toxic cytostatic micropollutants. Sci. Rep. 2020, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Himanshu, A.; Divyanshi, S.; Kumar, S.S.; Sonika, T.; Saiqa, I. Removal of mercury from wastewater use of green adsorbents—A review. Electron. J. Environ. Agric. Food Chem. 2010, 9, 1551–1558. [Google Scholar]
- Shen, S.; Yang, J.; Liu, C.; Bai, R. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Pascu, D.E.; Miron, A.R.; Pascu-Neagu, M.; Nechifor, A.C.; Totu, E.E. Fabrication, characterization, and modelling of polysulfone composite membranes with enhanced adsorbent capabilities. Sep. Sci. Technol. 2016, 51, 2628–2638. [Google Scholar] [CrossRef]
- Asadian, M.; Onyshchenko, I.; Thukkaram, M.; Tabaei, P.S.E.; Van Guyse, J.; Cools, P.; Declercq, H.; Hoogenboom, R.; Morent, R.; De Geyter, N. Effects of a dielectric barrier discharge (DBD) treatment on chitosan/polyethylene oxide nanofibers and their cellular interactions. Carbohyd. Polym. 2018, 201, 402–415. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Caprarescu, S.; Ion-Ebrasu, D.; Soare, A.; Purcar, V.; Radu, A.L.; Sarbu, A.; Pascu, M.; Modrogan, C.; Dancila, A.M.; Deleanu, C. Removal of nickel ions from synthetic wastewater using copolymers/natural extract blend membranes. Rom. J. Phys. 2019, 64, 821. Available online: http://www.nipne.ro/rjp/2019_64_9-10/RomJPhys.64.821.PDF (accessed on 16 December 2019).
- Caprarescu, S.; Ianchis, R.; Radu, A.L.; Sarbu, A.; Somoghi, R.; Trica, B.; Alexandrescu, E.; Spataru, C.I.; Fierascu, R.; Ion-Ebrasu, D.; et al. Synthesis, characterization and efficiency of new organically modified montmorillonite polyethersulfone membranes for removal of zinc ions from wastewasters. Appl. Clay Sci. 2017, 137, 135–142. [Google Scholar] [CrossRef]
- Su, Y.-N.; Lin, W.-S.; Hou, C.-H.; Den, W. Performance of integrated membrane filtration and electrodialysis processes for copper recovery from wafer polishing wastewater. J. Water Process Eng. 2014, 4, 149–158. [Google Scholar] [CrossRef]
- Chekioua, A.; Delimi, R. Purification of H2SO4 of pickling bath contaminated by Fe(II) ions using electrodialysis process. Energy Procedia 2015, 74, 1418–1433. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.F.D.; Klein, W.C.; Bernardes, A.M.; Ferreira, J.Z. Evaluation of the electrodialysis process for the treatment of metal finishing wastewater. J. Braz. Chem. Soc. 2002, 13, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Lasheen, M.R.; El-Sherif, I.Y.; Tawfik, M.E.; El-Wakeel, S.T.; El-Shahat, M.F. Preparation and adsorption properties of nano magnetite chitosan films for heavy metal ions from aqueous solution. Mater. Res. Bull. 2016, 80, 344–350. [Google Scholar] [CrossRef]
- Li, N.; Zheng, J.; Hadi, P.; Yang, M.; Huang, X.; Ma, H.; Walker, H.W.; Hsiao, B.S. Synthesis and characterization of a high flux nanocellulose–cellulose acetate nanocomposite membrane. Membranes 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Duranceau, S.J. Study of the effect of nanoparticles and surface morphology on reverse osmosis and nanofiltration membrane productivity. Membranes 2013, 3, 196–225. [Google Scholar] [CrossRef] [Green Version]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindra, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; Raju, K.M. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.F.; Wiesner, M. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Chen, G.; Lee, D.J. Synthesis of asymmetrical cellulose acetate/cellulose triacetate forward osmosis membrane: Optimization. J. Taiwan Inst. Chem. Eng. 2018, 96, 299–304. [Google Scholar] [CrossRef]
- Ghaee, A.; Shariaty-Niassar, M.; Barzin, J.; Matsuura, T.; Ismail, A.F. Preparation of chitosan/cellulose acetate composite nanofiltration membrane for wastewater treatment. Desalin. Water Treat. 2016, 57, 14453–14460. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Xing, X.Y.; Yu, Y.; Gu, L.; Xu, Z.K. Novel thin film composite membranes supported by cellulose triacetate porous substrates for high-performance forward osmosis. Polymer 2018, 153, 150–160. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, H.; Yang, C. Chitosan membrane adsorber for low concentration copper ion removal. Carbohydr. Polym. 2016, 146, 274–281. [Google Scholar] [CrossRef]
- Kim, D.S. The removal by crab shell of mixed heavy metal ions in aqueous solution. Bioresour. Technol. 2003, 87, 355–357. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Chitosan-based polymer blends: Current status and applications. J. Chem. Soc. Pak. 2014, 36, 11–27. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Removal of Cu2+, Cd2+ and Ni2+ ions from aqueous solution using a novel chitosan/polyvinyl alcohol adsorptive membrane. Carbohydr. Polym. 2019, 210, 264–273. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe (III) and Cr (VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Neoh, H.M.; Jamal, R. Effective immobilization of silver nanoparticles on a regenerated cellulose–chitosan composite membrane and its antibacterial activity. New J. Chem. 2017, 41, 5061–5065. [Google Scholar] [CrossRef]
- Reiad, N.A.; Salam, O.E.A.; Abadir, E.F.; Harraz, F.A. Adsorptive removal of iron and manganese ions from aqueous solutions with microporous chitosan/polyethylene glycol blend membrane. J. Environ. Sci. 2012, 24, 1425–1432. [Google Scholar] [CrossRef]
- Xiong, X.; Duan, J.; Zou, W.; He, X.; Zheng, W. A pH-sensitive regenerated cellulose membrane. J. Membr. Sci. 2010, 363, 96–102. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Zhao, C.; Zhao, B.; Dong, H.; Ma, S.; Chen, L.; Zhang, B. Cross-flow catalysis behavior of a PVDF/SiO2@Ag nanoparticles composite membrane. Polymers 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdorovets, M.V.; Korolkov, I.V.; Yeszhanov, A.B.; Gorin, Y.G. Functionalization of PET track-etched membranes by UV-induced graft (co)polymerization for detection of heavy metal ions in water. Polymers 2019, 11, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-W.; Park, J.; Pak, C.; Choi, K.; Lee, J.-C.; Chang, H. Design and synthesis of cross-linked copolymer membranes based on poly(benzoxazine) and polybenzimidazole and their application to an electrolyte membrane for a high-temperature PEM fuel cell. Polymers 2013, 5, 77–111. [Google Scholar] [CrossRef] [Green Version]
- Totu, E.E.; Ruse, E.; Luca, C. Modelling the electrochemical behavior of some ion-selective membranes by the impedance spectroscopy in alternating current. Rev. Chim. 2000, 59, 331–336. [Google Scholar]
- Totu, E.E.; Voicila, E.; Pistritu, V.; Cristache, C.; Nechifor, G. Evaluation of electrical characteristics for PMMA-TiO2 nanocomposites used in dentistry. Rev. Chim. 2018, 69, 155–159. [Google Scholar] [CrossRef]
- Totu, E.E.; Isildak, I.; Nechifor, A.C.; Cristache, C.M.; Enachescu, M. New sensor based on membranes with magnetic nano-inclusions for early diagnosis in periodontal disease. Biosens. Bioelectron. 2018, 102, 336–344. [Google Scholar] [CrossRef]
- Apetroaei, M.R.; Zgarian, R.G.; Manea, A.M.; Rău, I.; Tihan, G.T.; Schroder, V. New source of chitosan from Black Sea marine organisms identification. Mol. Cryst. Liq. Cryst. 2016, 628, 102–109. [Google Scholar] [CrossRef]
- Totu, E.E.; Ruse, E.; Girdea, R.; Grisorescu, A. FT-IR analysis on polyimide selective membranes. Optoelectron. Adv. Mater.-Rapid Commun. 2008, 2, 442–445. [Google Scholar]
- Sudha, P.N.; Vinodhini, P.A.; Sangeetha, K.; Latha, S.; Gomathi, T.; Venkatesan, J.; Kim, S.K. Fabrication of cellulose acetate-chitosan-polyethylene glycol ultrafiltration membrane for chromium removal. Der Pharm. Lett. 2014, 6, 37–46. Available online: http://scholarsresearchlibrary.com/archive.html (accessed on 10 February 2020).
- Ibrahim, M.M.; Fahmy, T.Y.A.; Salaheldin, E.I.; Mobarak, F.; Youssef, M.A.; Mabrook, M.R. Role of tosyl cellulose acetate as potential carrier for controlled drug release. Life Sci. J. 2015, 12, 127–133. [Google Scholar] [CrossRef]
- Ali, M.; Zafar, M.; Jamil, T.; Butt, M.T.Z. Influence of glycol additives on the structure and performance of cellulose acetate/zinc oxide blend membranes. Desalination 2011, 270, 98–104. [Google Scholar] [CrossRef]
- Rupiasih, N.N.; Sumadiyasa, M.; Putra, I.K.; Rasmini, N.M. Study on transport properties of chitosan membrane in different types of electrolytes. J. Math. Fund. Sci. 2018, 50, 182–191. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lee, S.J.; Moon, J.H.; Kim, J.H.; Heo, D.N.; Bang, J.B.; Limb, H.N.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact angle measurement on rough surfaces. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K. The influence on surface free energy of PVD-coatings. Surf. Coat. Technol. 2001, 142–144, 755–760. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Ghalloussi, R.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Structure and properties of heterogeneous and homogeneous ion-exchange membranes subjected to ageing in sodium hypochlorite. J. Membr. Sci. 2014, 452, 104–116. [Google Scholar] [CrossRef]
- Totu, E.; Segal, E.; Covington, A.K. On the thermal behavior of some polyimide membranes. J. Therm. Anal. Calorim. 1998, 52, 383–391. [Google Scholar] [CrossRef]
- Ahamed, M.I.N.; Sankara, S.; Kashif, P.M.; Basha, S.K.H.; Sastry, T.P. Evaluation of biomaterial containing regenerated cellulose and chitosan incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2015, 72, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef] [PubMed]
- Bharati, D.C.; Kumar, H.; Saroj, A.L. Chitosan-PEG-NaI based biopolymer electrolytes: Structural, thermal and ion dynamics studies. Mater. Res. Express 2020, 6, 125360. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Jalal, V.J.; Tahir, D.A.; Mohamad, A.H.; Abdullah, O.G. Effect of PEG as a plasticizer on the electrical and optical properties of polymer blend electrolyte MC-CH-LiBF4 based films. Results Phys. 2019, 15, 102735. [Google Scholar] [CrossRef]
- Dante, R.C. 9-Metals. In Handbook of Friction Materials and Their Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 123–134. [Google Scholar] [CrossRef]
- Mishra, P.C.; Islam, M.; Patel, R.K. Removal of lead (II) by chitosan from aqueous medium. Sep. Sci. Technol. 2013, 48, 1234–1242. [Google Scholar] [CrossRef]










| Liquid | Contact Angle (°) | |
|---|---|---|
| CA/PEG Membrane | CA/PEG/CHI-Ag Membrane | |
| Water | 101 ± 1.12 | 90.12 ± 0.62 |
| Dimethyl sulfoxide | 85 ± 0.72 | 60 ± 0.45 |
| Glycerol | 75 ± 0.68 | 74 ± 0.41 |
| Liquid | Surface Energies [mN/m] Owens-Wendt | ||
|---|---|---|---|
| γL | |||
| Water | 72.8 | 21.8 | 51 |
| Dimethyl sulfoxide | 44 | 36 | 8 |
| Glycerol | 63.4 | 37 | 26.4 |
| Membrane | mN m−1 | mN m−1 | mN m−1 |
|---|---|---|---|
| CA/PEG | 3.5280 | 14.7203 | 18.24828 |
| CA/PEG/CHI-Ag | 4.3786 | 21.6942 | 26.07273 |
| Membrane | 35–120 °C | 120–202 °C | 202–251.5 °C | 251.5–457.5 °C | 457.5–740 °C | Residue | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt. loss | Wt. Loss | Tmax | Wt. Loss | Tmax | Wt. Loss | Tmax | Wt. Loss | Tmax | 740 °C | |
| % | % | °C | % | °C | % | °C | % | °C | % | |
| CA/PEG | 3.03 | 1.32 | 181.3 | 1.93 | 231.3 | 79.20 | 359.6 | 14.41 | 583.2 | 0.11 |
| CA/PEG/CHI-Ag | 2.36 | 1.56 | 194.5 | 1.76 | 229.8 | 81.00 | 367.5 | 11.92 | 625.9 | 1.40 |
| Applied Voltage (V) | CA/PEG Membrane | CA/PEG/CHI-Ag Membrane | ||||
|---|---|---|---|---|---|---|
| TR (%) | IE (%) | Wc (kWh/m3) | TR (%) | IE (%) | Wc (kWh/m3) | |
| 5 | 15.86 | 9.57 | 6.99 | 30.35 | 27.33 | 4.10 |
| 10 | 29.43 | 8.07 | 29.88 | 41.09 | 25.56 | 10.13 |
| 15 | 33.71 | 7.71 | 48.73 | 63.70 | 9.42 | 37.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Eftimie Totu, E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers 2020, 12, 1792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081792
Căprărescu S, Zgârian RG, Tihan GT, Purcar V, Eftimie Totu E, Modrogan C, Chiriac A-L, Nicolae CA. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers. 2020; 12(8):1792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081792
Chicago/Turabian StyleCăprărescu, Simona, Roxana Gabriela Zgârian, Graţiela Teodora Tihan, Violeta Purcar, Eugenia Eftimie Totu, Cristina Modrogan, Anita-Laura Chiriac, and Cristian Andi Nicolae. 2020. "Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal" Polymers 12, no. 8: 1792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081792