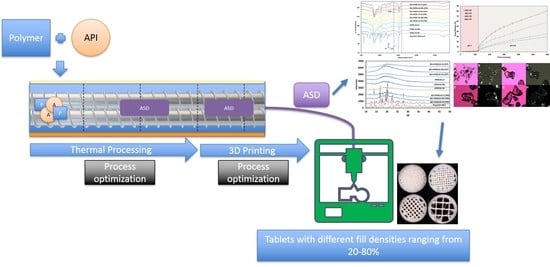
Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot-Melt Extrusion Process
2.3. Solid-State Characterization
2.3.1. Thermal Investigation
2.3.2. Powder X-ray Diffraction Studies
2.3.3. Polarized Light Microscopy
2.3.4. Fourier Transform–Infrared (FT–IR) Spectroscopic Analysis
2.4. Texture Analysis
2.5. Fused Deposition Modeling-Based 3-Dimensional Printing
2.6. Dosage form Performance (In Vitro Drug Release Testing)
2.7. Method of Analysis
2.8. Statistical Test and Dissolution Kinetics
3. Results
3.1. Preparation of Drug-Loaded FDM Filaments via HME
3.2. Solid-State Characterization
3.3. Texture Analysis
3.4. Morphological Characterization
3.5. In Vitro Drug Release Testing
3.6. Statistical Test and Dissolution Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Vogenberg, F.R.; Isaacson Barash, C.; Pursel, M. Personalized Medicine. Pharm. Ther. 2010, 35, 560–576. [Google Scholar]
- Lepowsky, E.; Tasoglu, S. 3D Printing for Drug Manufacturing: A Perspective on the Future of Pharmaceuticals. Int. J. Bioprinting 2018, 4, 119. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Cheah, C.M.; Leong, K.F.; Chua, C.K.; Low, K.H.; Quek, H.S. Characterization of microfeatures in selective laser sintered drug delivery devices. Proc. Inst. Mech. Eng. Part H 2016. [Google Scholar] [CrossRef] [PubMed]
- Low, K.H.; Leong, K.F.; Chua, C.K.; Du, Z.H.; Cheah, C.M. Characterization of SLS parts for drug delivery devices. Rapid Prototyp. J. 2001, 7, 262–268. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. Binder Jet Printing in Pharmaceutical Manufacturing. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–54. [Google Scholar] [CrossRef]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical Applications of Hot-Melt Extrusion: Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, H.E.; Tort, S.; Acartürk, F. An Effective Technology for the Development of Immediate Release Solid Dosage Forms Containing Low-Dose Drug: Fused Deposition Modeling 3D Printing. Pharm. Res. 2019, 36, 128. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline Caplets (PrintCap) via FDM 3D Printing. Polymers 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, S.; Park, J.-B.; Sohn, D.H.; Lee, S. Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.N.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Barnes, M.; McMillin, R.; Cook, D.W.; Smith, S.; Halquist, M.; Wijesinghe, D.; Roper, T.D. 3D Printing of Metformin HCl PVA Tablets by Fused Deposition Modeling: Drug Loading, Tablet Design, and Dissolution Studies. AAPS PharmSciTech 2019, 20, 195. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef]
- Dugar, R.P.; Gajera, B.Y.; Dave, R.H. Fusion Method for Solubility and Dissolution Rate Enhancement of Ibuprofen Using Block Copolymer Poloxamer 407. AAPS PharmSciTech 2016, 17, 1428–1440. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Al-Hamidi, H.; Adebisi, A.O.; Asare-Addo, K.; Maniruzzaman, M. The use of various organic solvents to tailor the properties of ibuprofen–glucosamine HCl solid dispersions. Chem. Eng. Res. Des. 2017, 117, 509–519. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Langlois, M.-H.; Dallet, P.; Kauss, T.; Dubost, J.-P. Simultaneous Determination of Ibuprofen and Pseudoephedrine Hydrochloride in Pharmaceutical Tablets by Reversed-Phase HPLC. Anal. Lett. 2009, 42, 2951–2961. [Google Scholar] [CrossRef]
- Yuksel, N.; Kanık, A.E.; Baykara, T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and -independent methods. Int. J. Pharm. 2000, 209, 57–67. [Google Scholar] [CrossRef]
- Kitak, T.; Dumičić, A.; Planinšek, O.; Šibanc, R.; Srčič, S. Determination of Solubility Parameters of Ibuprofen and Ibuprofen Lysinate. Molecules 2015, 20, 21549–21568. [Google Scholar] [CrossRef]
- Klar, F.; Urbanetz, N.A. Solubility parameters of hypromellose acetate succinate and plasticization in dry coating procedures. Drug Dev. Ind. Pharm. 2016, 42, 1621–1635. [Google Scholar] [CrossRef]
- Mallick, S.; Pattnaik, S.; Swain, K.; De, P.K.; Saha, A.; Mazumdar, P.; Ghoshal, G. Physicochemical Characterization of Interaction of Ibuprofen by Solid-State Milling with Aluminum Hydroxide. Drug Dev. Ind. Pharm. 2008, 34, 726–734. [Google Scholar] [CrossRef]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Dedroog, S.; Pas, T.; Vergauwen, B.; Huygens, C.; Van den Mooter, G. Solid-state analysis of amorphous solid dispersions: Why DSC and XRPD may not be regarded as stand-alone techniques. J. Pharm. Biomed. Anal. 2020, 178, 112937. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Characterization of amorphous solid dispersions: An update. J. Drug Deliv. Sci. Technol. 2019, 50, 113–124. [Google Scholar] [CrossRef]
- Davis, D.A.; Miller, D.A.; Williams, R.O. Thermally conductive excipient expands kinetiSol® processing capabilities. AAPS PharmSciTech 2020, in press. [Google Scholar]
- Xu, W.; Sun, Y.; Dong, X.; Li, S.; Wang, H.; Xue, J.; Zheng, X. Local order and vibrational coupling of the C=O Stretching Mode of γ -Caprolactone in liquid binary mixtures. Sci. Rep. 2017, 7, 12182. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Ashour, E.A.; Shukla, A.; Wang, R.; Chambliss, W.G.; Bandari, S.; Murthy, N.; Repka, M.A. A Comparison between Lab-Scale and Hot-Melt-Extruder-Based Anti-inflammatory Ointment Manufacturing. AAPS PharmSciTech 2020, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zemlyanov, D.; Chen, X.; Nie, H.; Su, Z.; Fang, K.; Yang, X.; Smith, D.; Byrn, S.; Lubach, J.W. Acid–Base Interactions of Polystyrene Sulfonic Acid in Amorphous Solid Dispersions Using a Combined UV/FTIR/XPS/ssNMR Study. Mol. Pharm. 2016, 13, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, D.E.; Zhang, G.G.Z.; Zhou, D.; Gao, Y.; Taylor, L.S. Understanding the Behavior of Amorphous Pharmaceutical Systems during Dissolution. Pharm. Res. 2010, 27, 608–618. [Google Scholar] [CrossRef]
- Ewing, A.V.; Clarke, G.S.; Kazarian, S.G. Stability of indomethacin with relevance to the release from amorphous solid dispersions studied with ATR-FTIR spectroscopic imaging. Eur. J. Pharm. Sci. 2014, 60, 64–71. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the Release Kinetics of a Pharmacologically Active Substance from Model Intra-Articular Implants Replacing the Cruciate Ligaments of the Knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Nukala, P.K.; Palekar, S.; Patki, M.; Patel, K. Abuse Deterrent Immediate Release Egg-Shaped Tablet (Egglets) Using 3D Printing Technology: Quality by Design to Optimize Drug Release and Extraction. AAPS PharmSciTech 2019, 20, 80. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T.M. Development of 3D Printed Tablets by Fused Deposition Modeling Using Polyvinyl Alcohol as Polymeric Matrix for Rapid Drug Release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Que, C.; Lou, X.; Zemlyanov, D.Y.; Mo, H.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Behavior of Ledipasvir–Copovidone Amorphous Solid Dispersions: Role of Drug Loading and Intermolecular Interactions. Mol. Pharm. 2019, 16, 5054–5067. [Google Scholar] [CrossRef]










| Parameters | Values |
|---|---|
| Zones | 4 |
| Temperature (°C) | 130 °C throughout |
| Screw RPM | 50 |
| * Feed rate (RPM) | 500 ± 50 (5 g/min) |
| Observed torque on equilibration | 5.30 ± 0.27 N·m |
| Residence time (min) | 2.3 ± 0.2 |
| Die pressure | 55 ± 10 psi |
| Print Parameters | Values Set |
|---|---|
| Print temperature | 175 °C–180 °C |
| Bed temperature | 60 °C |
| Print speed | 70 mm/s |
| Layer height | 0.1 mm |
| Wall thickness | 2 mm |
| Number of walls | 2 |
| Fan speed | 70% |
| Infill pattern | Lines |
| Top/bottom layers | None |
| Print core | AA 0.4 |
| Result Parameters | Hardness (Kp) | Brittleness/Flexibility, Travel Distance (mm) | Gradient (MPa/mm) |
|---|---|---|---|
| 20% (w/w) ibuprofen + HPMC-AS HG | |||
| Average | 3.99 | 2.09 | 3.582 |
| Standard Deviation (S.D.) | 0.76 | 0.06 | 0.715 |
| Coefficient of Variance (C.V.) | 18.94 | 3.1 | 19.963 |
| 20% (w/w) ibuprofen + HPMC-AS MG | |||
| Average | 2.11 | 2.16 | 2.445 |
| Standard Deviation (S.D.) | 0.30 | 0.03 | 0.057 |
| Coefficient of Variance (C.V.) | 14.3 | 1.55 | 2.339 |
| 20% (w/w) ibuprofen + HPMC-AS LG | |||
| Average | 1.51 | 2.31 | 2.179 |
| Standard Deviation (S.D.) | 0.21 | 0.23 | 0.153 |
| Coefficient of Variance (C.V.) | 13.91 | 10.05 | 7.02 |
| Tough PLA filaments | |||
| Average | 5.05 | 1.84 | 6.269 |
| Standard Deviation (S.D.) | 0.13 | 0.07 | 0.204 |
| Coefficient of Variance (C.V.) | 2.65 | 3.55 | 3.258 |
| Natural PVA filaments | |||
| Average | 1.05 | 3.99 | 0.607 |
| Standard Deviation (S.D.) | 0.24 | 0.36 | 0.189 |
| Coefficient of Variance (C.V.) | 22.44 | 9.02 | 31.1 |
| Time Point | Result |
|---|---|
| 125 | F (11, 24) = 1016.780, p < 0.0001 |
| 130 | F (11, 24) = 21,291.52, p < 0.0001 |
| 135 | F (11, 24) = 3926.784, p < 0.0001 |
| 150 | F (11, 24) = 28,952.28, p < 0.0001 |
| 180 | F (11, 24) = 376.868, p < 0.0001 |
| 210 | F (11, 24) = 25,253.55, p < 0.0001 |
| 240 | F (11, 24) = 20,503.98, p < 0.0001 |
| 300 | F (11, 24) = 13,697.65, p < 0.0001 |
| 360 | F (11, 24) = 13,738.84, p < 0.0001 |
| 420 | F (11, 24) = 11,424.00, p < 0.0001 |
| 480 | F (11, 24) = 15,084.65, p < 0.0001 |
| Formulation Compositions (20% w/w Ibuprofen) | Kinetic Models (R2) | ||||||
|---|---|---|---|---|---|---|---|
| % Infill | Zero-Order Kinetics | First-Order Kinetics | Higuchi Model | Korsmeyer–Peppas | Hixson–Crowell | Weibull Function | |
| HPMC-AS HG | 20 | 0.9522 | 0.7795 | 0.9992 | 0.9982 | 0.8497 | 0.9859 |
| 40 | 0.9704 | 0.7604 | 0.9784 | 0.9955 | 0.8546 | 0.9934 | |
| 60 | 0.9679 | 0.7915 | 0.9765 | 0.9947 | 0.8661 | 0.9926 | |
| 80 | 0.9703 | 0.815 | 0.9928 | 0.9928 | 0.8809 | 0.9899 | |
| HPMC-AS MG | 20 | 0.8587 | 0.6807 | 0.7353 | 0.9758 | 0.7549 | 0.9136 |
| 40 | 0.959 | 0.7183 | 0.6195 | 0.9904 | 0.8347 | 0.9004 | |
| 60 | 0.9683 | 0.7487 | 0.5841 | 0.9964 | 0.8558 | 0.8915 | |
| 80 | 0.9759 | 0.6322 | 0.4707 | 0.9404 | 0.828 | 0.9679 | |
| HPMC-AS LG | 20 | 0.9978 | 0.9456 | 0.6613 | 0.9942 | 0.9812 | 0.9415 |
| 40 | 0.935 | 0.8492 | 0.514 | 0.9754 | 0.8973 | 0.9352 | |
| 60 | 0.8884 | 0.6397 | 0.5128 | 0.9634 | 0.7425 | 0.9869 | |
| 80 | 0.9171 | 0.6556 | 0.5979 | 0.9706 | 0.7648 | 0.9885 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakkar, R.; Pillai, A.R.; Zhang, J.; Zhang, Y.; Kulkarni, V.; Maniruzzaman, M. Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool. Polymers 2020, 12, 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091872
Thakkar R, Pillai AR, Zhang J, Zhang Y, Kulkarni V, Maniruzzaman M. Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool. Polymers. 2020; 12(9):1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091872
Chicago/Turabian StyleThakkar, Rishi, Amit Raviraj Pillai, Jiaxiang Zhang, Yu Zhang, Vineet Kulkarni, and Mohammed Maniruzzaman. 2020. "Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool" Polymers 12, no. 9: 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091872