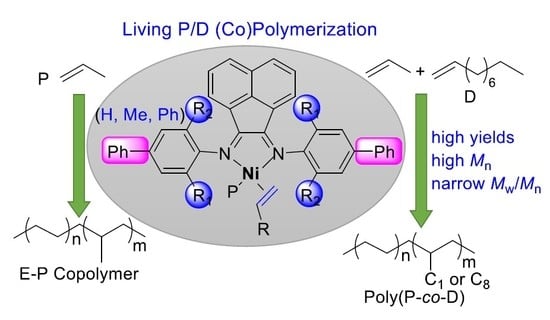
Living Chain-Walking (Co)Polymerization of Propylene and 1-Decene by Nickel α-Diimine Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. General Considerations
2.2. Polymerization Procedure
2.3. Time-Course of Propylene and 1-Decene Copolymerization
3. Results and Discussion
3.1. Synthesis and Characterization of the Nickel Complexes
3.2. Catalytic Polymerization of Propylene
3.3. Copolymerization of Propylene and 1-Decene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takeuchi, D.; Osakada, K. Controlled isomerization polymerization of olefins, cycloolefins, and dienes. Polymer 2016, 82, 392–405. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α-diimine catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C.L. Emerging palladium and nickel catalysts for copolymerization of olefins with polar monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Gahleitner, M.; Paulik, C. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Chen, Y.; Wang, L.; Yu, H.; Zhao, Y.; Sun, R.; Jing, G.; Huang, J.; Khalid, H.; Abbasi, N.M.; Akram, M. Synthesis and application of polyethylenebased functionalized hyperbranched polymers. Prog. Polym. Sci. 2015, 45, 23–43. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.L. Direct and Tandem Routes for the Copolymerization of Ethylene with Polar Functionalized Internal Olefins. Angew. Chem. Int. Ed. 2020, 59, 1206–1210. [Google Scholar] [CrossRef]
- Dai, S.; Li, S.; Xu, G.; Chen, C.L. Direct Synthesis of Polar Functionalized Polyethylene Thermoplastic Elastomer. Macromolecules 2020, 53, 2539–2546. [Google Scholar] [CrossRef]
- Na, Y.N.; Chen, C.L. Catechol Functionalized Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. 2-Phosphine-pyridine-N-oxide palladium and nickel catalysts for ethylene polymerization and copolymerization with polar monomers. Polymer 2020, 194, 122410. [Google Scholar] [CrossRef]
- Tan, C.; Qasim, M.; Pang, W.M.; Chen, C.L. Ligand-Metal Secondary Interaction in Phosphine-Sulfonate Palladium and Nickel Catalyzed Ethylene (Co)Polymerization. Polym. Chem. 2020, 11, 411–416. [Google Scholar] [CrossRef]
- Xu, M.L.; Yu, F.; Li, P.; Xu, G.; Zhang, S.J.; Wang, F.Z. Enhancing Chain Initiation Efficiency in the Cationic Allyl-Nickel Catalyzed (Co)Polymerization of Ethylene and Methyl Acrylate. Inorg. Chem. 2020, 59, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, C.L. Polar-Functionalized, Crosslinkable, Self-Healing and Photoresponsive Polyolefins. Angew. Chem. Int. Ed. 2020, 59, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Goudari, S.; Chen, C.L. A Simple and Versatile Nickel Platform for the Generation of Branched High Molecular Weight Polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, Q.; Tan, C.; Chen, C.L. Concerted steric and electronic effects on α-diimine nickel- and palladium-catalyzed ethylene polymerization and copolymerization. Sci. Bull. 2020, 65, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cheng, H.; Xiao, R.; Cai, Z. Rational design of nickel catalysts containing N-acylated imidazolin-2-imine ligand for ethylene copolymerization with polar monomer. J. Catal. 2020, 383, 117–123. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.L. A versatile ligand platform for palladium- and nickel-catalyzed ethylene copolymerization with polar monomers. Angew. Chem. Int. Ed. 2018, 57, 3094–3098. [Google Scholar] [CrossRef]
- Dai, S.Y.; Chen, C.L. A Self-Supporting Strategy for Gas-Phase and Slurry-Phase Ethylene Polymerization using Late-Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2020, 59, 2–9. [Google Scholar] [CrossRef]
- Zhou, S.X.; Chen, C.L. Synthesis of silicon-functionalized polyolefins by subsequent cobalt-catalyzed dehydrogenative silylation and nickel-catalyzed copolymerization. Sci. Bull. 2018, 63, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Kocen, A.L.; Brookhart, M.; Daugulis, O. A highly active Ni(II)-triadamantylphosphine catalyst for ultrahigh-molecular-weight polyethylene synthesis. Nat. Chem. 2019, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Na, Y.N.; Dai, S.Y.; Chen, C.L. Direct synthesis of polar-functionalized linear low-density polyethylene (LLDPE) and low-density polyethylene (LDPE). Macromolecules 2018, 51, 4040–4048. [Google Scholar] [CrossRef]
- Figueira, C.A.; Lopes, P.S.; Gomes, C.S.B.; Gomes, J.C.S.; Veiros, L.F.; Lemos, F.; Gomes, P.T. Neutral mono(5-aryl-2-iminopyrrolyl)nickel(II) complexes as precatalysts for the synthesis of highly branched ethylene oligomers: Preparation, molecular characterization, and catalytic studies. Organometallics 2019, 38, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.A.; Anderson, W.C.; Mitchell, N.E.; Gmernicki, K.R.; Long, B.K. High Temperature, Living polymerization of ethylene by a sterically-demanding nickel(II) α-diimine catalyst. Polymers 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Chen, C.L. Influence of polyethylene glycol unit on palladium- and nickel-catalyzed ethylene polymerization and copolymerization. Angew. Chem. Int. Ed. 2017, 56, 14672–14676. [Google Scholar] [CrossRef]
- Hu, H.; Gao, H.; Chen, D.; Li, G.; Tan, Y.; Liang, G.; Zhu, F.; Qing, W. Ligand-directed regioselectivity in amine-imine nickel-catalyzed 1-hexene polymerization. ACS Catal. 2015, 5, 122–128. [Google Scholar] [CrossRef]
- Ma, X.; Hu, X.; Zhang, Y.; Mu, H.; Cui, L.; Jian, Z.B. Preparation and in situ chain-end-functionalization of branched ethylene oligomers by monosubstituted α-diimine nickel catalysts. Polym. Chem. 2019, 10, 2596–2607. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tian, S.S.; Li, R.P.; Li, W.M.; Chen, C.L. Ligand steric effects on naphthyl-α-diimine nickel catalyzed α-olefin polymerization. Chinese J. Polym. Sci. 2018, 36, 157–162. [Google Scholar] [CrossRef]
- Gao, H.; Liu, X.; Tang, Y.; Pan, J.; Wu, Q. Living/controlled polymerization of 4-methyl-1-pentene with α-diimine nickel-diethylaluminium chloride: Effect of alkylaluminium cocatalysts. Polym. Chem. 2011, 2, 1398–1403. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Zhong, L.; Du, C.; Liao, H.; Gao, H.; Wu, Q. Living isomerization polymerizations of alkenylcyclohexane with camphyl α-diimine nickel catalysts. Polymer 2019, 164, 26–32. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tanaka, R.; Cai, Z.G.; Nakayama, Y.; Shiono, T. Precision chain-walking polymerization of trans-4-octene catalyzed by α-diimine nickel(II) catalysts bearing ortho-sec-phenethyl groups. Macromol. Rapid Commun. 2016, 37, 1375–1381. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tanaka, R.; Cai, Z.G.; Nakayama, Y.; Shiono, T. Living polymerization of higher 2-alkene with α-diimine nickel catalysts: Synthesis and characterization of high molecular weight poly(2-alkene)s. Polymer 2017, 127, 88–100. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tanaka, R.; Li, Q.S.; Nakayama, Y.; Shiono, T. Chain-walking polymerization of linear internal octenes catalyzed by α-diimine nickel complexes. Organometallics 2018, 37, 1358–1367. [Google Scholar] [CrossRef]
- Wang, F.Z.; Xu, G.Y.; Li, Q.S.; Tanaka, R.; Nakayama, Y.; Shiono, T. Chain-walking polymerization of 3-heptene with phenyl substituted α-diimine nickel catalysts. Polymer 2019, 181, 121801. [Google Scholar] [CrossRef]
- Zhang, D.F.; Nadres, E.T.; Brookhart, M.; Daugulis, O. Synthesis of highly branched polyethylene using “sandwich” (8-p-tolyl naphthyl α-diimine) nickel(II) catalysts. Organometallics 2013, 32, 5136–5143. [Google Scholar] [CrossRef]
- Wang, X.X.; Fan, L.L.; Ma, Y.P.; Guo, C.Y.; Solan, G.A.; Sun, Y.; Sun, W.H. Elastomeric polyethylenes accessible via ethylene homo-polymerization using an unsymmetrical α-diimino-nickel catalyst. Polym. Chem. 2017, 8, 2785–2795. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, X.B.; Luo, Y.; Chen, C.L. A second-coordination-sphere strategy to modulate nickel- and palladium-catalyzed olefin polymerization and copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef]
- Lian, K.B.; Zhu, Y.; Li, W.M.; Dai, S.Y.; Chen, C.L. Direct synthesis of thermoplastic polyolefin elastomers from nickel-catalyzed ethylene polymerization. Macromolecules 2017, 50, 6074–6080. [Google Scholar] [CrossRef]
- Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. Synthesis of polyethylenes with controlled branching with α-diimine nickel catalysts and revisiting formation of long-chain branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.L.; Li, Y.G.; Chen, C.L. Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 9, 4143–4149. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Cui, L.; Mu, H.; Jian, Z.B. Pentiptycenyl substituents in insertion polymerization with α-diimine nickel and palladium species. Organometallics 2019, 389, 2075–2083. [Google Scholar] [CrossRef]
- Tan, C.; Pang, W.; Chen, C.L. A phenol-containing α-diimine ligand for nickel- and palladium-catalyzed ethylene polymerization. Chinese J. Polym. Sci. 2019, 37, 974–980. [Google Scholar] [CrossRef]
- Chen, C.L. Redox-controlled polymerization and copolymerization. ACS Catal. 2018, 8, 5506–5514. [Google Scholar] [CrossRef]
- Wang, F.Z.; Chen, C.L. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Canetti, M.; Pierro, I.; Zanchin, G.; Rosa, C.D.; Ricci, G.; Bertini, F. (Micro)structure, thermal behavior and mechanical properties of ethylene-propylene-1-octadecene terpolymers from chain-walking polymerization of 1-octadecene. Polymer 2019, 166, 27–37. [Google Scholar] [CrossRef]
- Zambelli, A.; Sessa, I.; Grisi, F.; Fusco, R.; Accomazzi, P. Syndiotactic polymerization of propylene: Single-site vanadium catalysts in comparison with zirconium and nickel. Macromol. Rapid Commun. 2001, 22, 297–310. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.R.; Wu, H.; Xiao, Z.F.; Gao, H.Y.; Zhu, F.M.; Wu, Q. Polymerization of α-olefins using a camphyl α-diimine nickel catalyst at elevated temperature. Macromolecules 2014, 47, 3325–3331. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, X.; Gong, X.; Xu, D.; Ma, Y. Macrocyclic trinuclear nickel phenoxyimine catalysts for high-temperature polymerization of ethylene and isospecific polymerization of propylene. Macromolecules 2017, 50, 6561–6568. [Google Scholar] [CrossRef]
- O’Connor, K.S.; Lamb, J.R.; Vaidya, T.; Keresztes, I.; Klimovica, K.; LaPointe, A.M.; Daugulis, O.; Coates, G.W. Understanding the insertion pathways and chain walking mechanisms of α-diimine nickel catalysts for α-olefin polymerization: A 13C NMR spectroscopic investigation. Macromolecules 2017, 50, 7010–7027. [Google Scholar] [CrossRef]
- Vaccarello, D.N.; O’Connor, K.S.; Iacono, P.; Rose, J.M.; Cherian, A.E.; Coates, G.W. Synthesis of semicrystalline polyolefin materials: Precision methyl branching via stereoretentive chain walking. J. Am. Chem. Soc. 2018, 140, 6208–6211. [Google Scholar] [CrossRef]
- Vaidya, T.; Klimovica, K.; LaPointe, A.M.; Keresztes, I.; Lobkovsky, E.B.; Daugulis, O.; Coates, G.W. Secondary alkene insertion and precision chain-walking: A new route to semicrystalline “polyethylene” from α-olefins by combining two rare catalytic events. J. Am. Chem. Soc. 2014, 136, 7213–7216. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tanaka, R.; Li, Q.S.; Yuan, J.C.; Nakayama, Y.; Shiono, T. Synthesis and application of α-diimine Ni(II) and Pd(II) complexes with bulky steric groups to polymerization of ethylene and methyl methacrylate. J. Mol. Catal. A Chem. 2015, 398, 231–240. [Google Scholar] [CrossRef]
- Wang, F.Z.; Li, R.P.; Tian, S.S.; Lian, K.B.; Guo, D.F.; Li, W.M. Synthesis of highly branched polyethylene using para-phenyl-substituted α-diimine nickel catalysts. Appl. Organomet. Chem. 2018, 32, 4298. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Q.; Xu, G.; Li, Y.F.; Wang, Z. Cationic para-phenyl-substituted α-diimine nickel catalyzed ethylene and 4-methyl-1-pentene (co)polymerizations via living/controlled chain-walking. Appl. Organomet. Chem. 2019, 33, e4911. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tanaka, R.; Cai, Z.G.; Nakayama, Y.; Shiono, T. Synthesis of highly branched polyolefins using phenyl substituted α-diimine Ni(II) catalysts. Polymers 2016, 8, 160. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, Y.G.; Li, Y.S.; Hu, N.H. Ethylene polymerization by (α-diimine) nickel(II) complexes bearing different substituents on para-position of imines activated with MMAO. J. Appl. Polymer. Sci. 2008, 109, 700–707. [Google Scholar] [CrossRef]
- McCord, E.F.; McLain, S.J.L.; Nelson, T.J.; Ittel, S.D.; Tempel, D.; Killian, C.M.; Johnson, L.K.; Brookhart, M. 13C NMR Analysis of α-olefin enchainment in poly(α-olefins) produced with nickel and palladium α-diimine catalysts. Macromolecules 2007, 40, 410–420. [Google Scholar] [CrossRef]
- Azoulay, J.D.; Bazan, G.C.; Galland, G.B. Microstructural characterization of poly(1-hexene) obtained using a nickel α-keto-β-diimine initiator. Macromolecules 2010, 43, 2794–2800. [Google Scholar] [CrossRef]
- Jeon, M.; Han, C.J.; Kim, S.Y. Polymerizations of propylene with unsymmetrical (α-diimine)nickel(2) catalysts. Macromol. Res. 2006, 14, 306–311. [Google Scholar] [CrossRef]
- Pellecchia, C.; Zambelli, A. Syndiotactic-specific polymerization of propene with a Ni-based catalyst. Macromol. Rapid Commun. 1996, 17, 333–338. [Google Scholar] [CrossRef]






| Entry | Cat. | Temp. (°C) | Time (min) | Yield (g) | Activity b | Mnc (×104) | Mw/Mnc | N d (μmol) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 0 | 30 | 0.18 | 36 | 9.5 | 1.06 | 1.9 |
| 2 | 2 | 25 | 30 | 0.43 | 85 | 13.4 | 1.16 | 3.2 |
| 3 | 2 | 50 | 30 | 0.35 | 70 | 12.2 | 1.56 | 2.9 |
| 4 | 1 | 25 | 30 | 0.40 | 80 | 11.9 | 1.24 | 3.4 |
| 5 | 3 | 25 | 30 | 0.37 | 74 | 14.2 | 1.31 | 2.6 |
| 6 | 4 | 25 | 30 | 0.25 | 50 | 19.1 | 1.48 | 1.3 |
| 7 | 5 | 25 | 30 | 0.11 | 22 | 8.1 | 1.41 | 1.4 |
| 8 | 2 | 25 | 10 | 0.14 | 84 | 4.4 | 1.19 | 3.2 |
| 9 | 2 | 25 | 20 | 0.30 | 90 | 9.4 | 1.10 | 3.2 |
| 10 | 2 | 25 | 40 | 0.52 | 78 | 16.9 | 1.16 | 3.1 |
| 11 | 2 | 25 | 50 | 0.63 | 76 | 21.3 | 1.25 | 3.0 |
| Entry | Cat. | Temp. (°C) | Time (min) | Branches a/1000 C | 1,3-Enchain. b (%) | Tgc (°C) |
|---|---|---|---|---|---|---|
| 1 | 2 | 0 | 30 | 267 | 48 | −24.1 |
| 2 | 2 | 25 | 30 | 253 | 50 | −28.8 |
| 7 | 5 | 25 | 30 | 266 | 48 | −28.3 |
| 8 | 2 | 25 | 10 | 263 | 48 | −28.4 |
| 9 | 2 | 25 | 20 | 259 | 49 | −28.6 |
| 10 | 2 | 25 | 40 | 249 | 50 | −29.4 |
| 11 | 2 | 25 | 50 | 245 | 51 | −29.9 |
| Entry | Cat. | Temp. (°C) | Yield (g) | Activity b | Mnc (×104) | Mw/Mnc | Branches d/1000 C | Tge (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 25 | 1.13 | 226 | 13.7 | 1.14 | 169 | −49.9 |
| 2 | 2 | 0 | 0.79 | 158 | 12.3 | 1.07 | 187 | −49.1 |
| 3 | 5 | 25 | 0.53 | 106 | 7.3 | 1.93 | 171 | −49.5 |
| Entry | Time (min) | Yield (g) | Activity b | Mnc (×104) | Mw/Mnc | N d (μmol) |
|---|---|---|---|---|---|---|
| 1 | 5 | 0.05 | 300 | 5.1 | 1.11 | 1.0 |
| 2 | 10 | 0.09 | 270 | 8.0 | 1.11 | 1.1 |
| 3 | 30 | 0.18 | 180 | 14.2 | 1.11 | 1.2 |
| 4 | 50 | 0.21 | 126 | 17.1 | 1.12 | 1.2 |
| 5 | 70 | 0.25 | 107 | 20.4 | 1.10 | 1.2 |
| 6 | 90 | 0.28 | 93 | 23.1 | 1.10 | 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Li, X.; Behzadi, S.; Xu, M.; Yu, F.; Xu, G.; Wang, F. Living Chain-Walking (Co)Polymerization of Propylene and 1-Decene by Nickel α-Diimine Catalysts. Polymers 2020, 12, 1988. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091988
Li P, Li X, Behzadi S, Xu M, Yu F, Xu G, Wang F. Living Chain-Walking (Co)Polymerization of Propylene and 1-Decene by Nickel α-Diimine Catalysts. Polymers. 2020; 12(9):1988. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091988
Chicago/Turabian StyleLi, Pei, Xiaotian Li, Shabnam Behzadi, Mengli Xu, Fan Yu, Guoyong Xu, and Fuzhou Wang. 2020. "Living Chain-Walking (Co)Polymerization of Propylene and 1-Decene by Nickel α-Diimine Catalysts" Polymers 12, no. 9: 1988. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091988