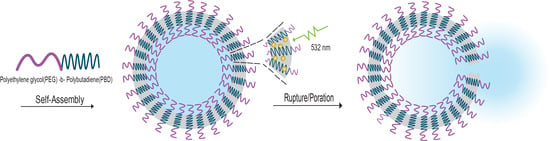
Polymersome Poration and Rupture Mediated by Plasmonic Nanoparticles in Response to Single-Pulse Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micron-Scale Polymersome Preparation
2.2. Polymersome Imaging
2.3. Removal of Excess Encapsulant
2.4. Pulsed Laser Irradiation
2.5. Studying the Fate of the Incorporated AuNPs upon Polymersome Irradiation
2.6. Nano-Scale Polymersome Preparation
2.7. Size Confirmation
3. Results
3.1. AuNPs as Photosensitizers for Complete Polymersome Rupture
3.2. Using Pulse Duration for Mechanistic Insight
3.3. Controlling Complete Rupture vs. Pore Formation with Decreased Pulse Energy
3.4. Role of AuNP Concentration on Vesicle Poration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoPresti, C.; Lomas, H.; Massignani, M.; Smart, T.; Battaglia, G. Polymersomes: Nature inspired nanometer sized compartments. J. Mater. Chem. 2009, 19, 3576–3590. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, H.; Hest, J. Stimuli-responsive polymersomes and nanoreactors. J. Mater. Chem. B 2016, 4, 4632–4647. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [Green Version]
- Cabane, E.; Malinova, V.; Meier, W. Synthesis of photocleavable amphiphilic block copolymers: Toward the design of photosensitive nanocarriers. Macromol. Chem. Phys. 2010, 211, 1847–1856. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, Y.; Xun, Z.; Han, Y.; Chen, J.; Li, Y.-Y.; Li, Y. Stabilized vesicles consisting of small amphiphiles for stepwise photorelease via uv light. Langmuir 2012, 28, 1733–1737. [Google Scholar] [CrossRef]
- Schumers, J.-M.; Fustin, C.-A.; Gohy, J.-F. Light-responsive block copolymers. Macromol. Rapid Commun. 2010, 31, 1588–1607. [Google Scholar] [CrossRef]
- Mabrouk, E.; Cuvelier, D.; Brochard-Wyart, F.; Nassoy, P.; Li, M.-H. Bursting of sensitive polymersomes induced by curling. Proc. Natl. Acad. Sci. USA 2009, 106, 7294–7298. [Google Scholar] [CrossRef] [Green Version]
- Robbins, G.P.; Jimbo, M.; Swift, J.; Therien, M.J.; Hammer, D.A.; Dmochowski, I.J. Photoinitiated destruction of composite porphyrin-protein polymersomes. J. Am. Chem. Soc. 2009, 131, 3872–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griepenburg, J.C.; Sood, N.; Vargo, K.B.; Williams, D.; Rawson, J.; Therien, M.J.; Hammer, D.A.; Dmochowski, I.J. Caging metal ions with visible light-responsive nanopolymersomes. Langmuir 2015, 31, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kamat, N.P.; Liao, Z.; Moses, L.E.; Rawson, J.; Therien, M.J.; Dmochowski, I.J.; Hammer, D.A. Sensing membrane stress with near ir-emissive porphyrins. Proc. Natl. Acad. Sci. USA 2011, 108, 13984–13989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Np, K.; Gp, R.; Js, R.; Mj, T.; Ij, D.; Da, H. A generalized system for photo-responsive membrane rupture in polymersomes. Adv. Funct. Mater. 2010, 20, 2588–2596. [Google Scholar] [CrossRef]
- Amstad, E.; Kim, S.-H.; Weitz, D.A. Photo- and thermoresponsive polymersomes for triggered release. Angew. Chem 2012, 51, 12499–12503. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, W.; Qu, X.; Wu, H.; Qu, L.; Zhang, X.; Mäkilä, E.; Salonen, J.; Zhu, Y.; Yang, Z.; et al. Photothermal-responsive nanosized hybrid polymersome as versatile therapeutics codelivery nanovehicle for effective tumor suppression. Proc. Natl. Acad. Sci. USA 2019, 116, 7744–7749. [Google Scholar] [CrossRef] [Green Version]
- Miranda, D.; Lovell, J.F. Mechanisms of light-induced liposome permeabilization. Bioeng. Transl. Med. 2016, 1, 267–276. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nano-Micro Lett. 2018, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mathiyazhakan, M.; Yang, Y.; Liu, Y.; Zhu, C.; Liu, Q.; Ohl, C.-D.; Tam, K.C.; Gao, Y.; Xu, C. Non-invasive controlled release from gold nanoparticle integrated photo-responsive liposomes through pulse laser induced microbubble cavitation. Colloids Surf. B 2015, 126, 569–574. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.M.; Bermudez, H.; Discher, B.M.; Sheehan, M.A.; Won, Y.Y.; Bates, F.S.; Discher, D.E. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol. Bioeng. 2001, 73, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.C.; Sasaki, D.Y.; Bachand, G.D. Forming giant-sized polymersomes using gel-assisted rehydration. J. Vis. Exp. 2016, e54051. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled eosin y doped pmma nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef]
- Habel, J.; Ogbonna, A.; Larsen, N.; Cherré, S.; Kynde, S.; Midtgaard, S.R.; Kinoshita, K.; Krabbe, S.; Jensen, G.V.; Hansen, J.S.; et al. Selecting analytical tools for characterization of polymersomes in aqueous solution. RSC Adv. 2015, 5, 79924–79946. [Google Scholar] [CrossRef] [Green Version]
- Metwally, K.; Mensah, S.; Baffou, G. Fluence threshold for photothermal bubble generation using plasmonic nanoparticles. J. Phys. Chem. C 2015, 119, 28586–28596. [Google Scholar] [CrossRef] [Green Version]
- Boulais, E.; Lachaine, R.; Hatef, A.; Meunier, M. Plasmonics for pulsed-laser cell nanosurgery: Fundamentals and applications. J. Photochem. Photobiol. C 2013, 17, 26–49. [Google Scholar] [CrossRef]
- Klein-Wiele, J.H.; Simon, P.; Rubahn, H.G. Size-dependent plasmon lifetimes and electron-phonon coupling time constants for surface bound na clusters. Phys. Rev. Lett. 1998, 80, 45–48. [Google Scholar] [CrossRef]
- Delfour, L.; Itina, T.E. Mechanisms of ultrashort laser-induced fragmentation of metal nanoparticles in liquids: Numerical insights. J. Phys. Chem. C 2015, 119, 13893–13900. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiSalvo, G.M.; Robinson, A.R.; Aly, M.S.; Hoglund, E.R.; O’Malley, S.M.; Griepenburg, J.C. Polymersome Poration and Rupture Mediated by Plasmonic Nanoparticles in Response to Single-Pulse Irradiation. Polymers 2020, 12, 2381. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102381
DiSalvo GM, Robinson AR, Aly MS, Hoglund ER, O’Malley SM, Griepenburg JC. Polymersome Poration and Rupture Mediated by Plasmonic Nanoparticles in Response to Single-Pulse Irradiation. Polymers. 2020; 12(10):2381. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102381
Chicago/Turabian StyleDiSalvo, Gina M., Abby R. Robinson, Mohamed S. Aly, Eric R. Hoglund, Sean M. O’Malley, and Julianne C. Griepenburg. 2020. "Polymersome Poration and Rupture Mediated by Plasmonic Nanoparticles in Response to Single-Pulse Irradiation" Polymers 12, no. 10: 2381. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102381