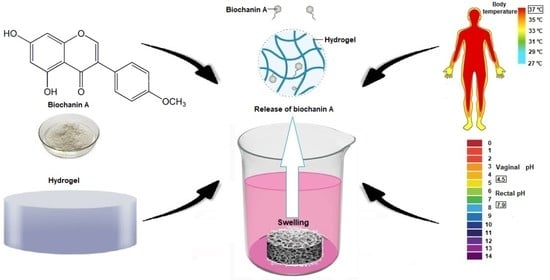
Modified Biochanin A Release from Dual pH- and Thermo-Responsive Copolymer Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Copolymeric p(NIPAM-co-AA) Hydrogel
2.3. Lyophilization of the Copolymeric p(NIPAM-co-AA) Hydrogel
2.4. Incorporation of the Biochanin A into the Copolymeric p(NIPAM-co-AA) Hydrogel
2.5. Modified Release of Biochanin A from the Copolymeric p(NIPAM-co-AA) Hydrogel
2.6. Characterization Methods
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.2. High-Pressure Liquid Chromatography (HPLC)
2.6.3. Swelling Study
2.6.4. Modelling the Process of p(NIPAM-co-AA) Copolymer Swelling
2.6.5. UV/Vis Spectrophotometry
2.6.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Synthesis of the Poly(N-Isopropylacrylamide-co-Acrylic Acid) Polymer
3.2. Structural Characterization of Synthesized Poly(N-Isopropylacrylamide-co-Acrylic Acid) Copolymer
3.2.1. FTIR Spectroscopy Analysis
3.2.2. Residual Reactant Analysis
3.2.3. Swelling Study
3.3. Examination of p(NIPAM-co-AA) Copolymer as a Matrix for Modified Release of Biochanin A
3.3.1. Structural Analysis of p(NIPAM-co-AA) Copolymer with Incorporated Biochanin A
3.3.2. Scanning Electron Microscopy Analysis
3.3.3. The Loading Efficiency of Biochanin A into the p(NIPAM-co-AA) Hydrogel
3.3.4. In Vitro Release of Biochanin A from p(NIPAM-co-AA) Copolymer
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breikaa, R.M.; Algandaby, M.M.; El-Demerdash, E.; Abdel-Naim, A.B. Multimechanistic antifibrotic effect of biochanin a in rats: Implications of proinflammatory and profibrogenic mediators. PLoS ONE 2013, 8, e69276. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, A.; Radhiga, T.; Deivasigamani, B. Biological activity of biochanin A: A review. Asian. J. Pharm. Pharmacol. 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Cao, W.; Huang, Y.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors. 2019, 45, 563–574. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar] [CrossRef]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Tech. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Moon, Y.J.; Sagawa, K.; Frederick, K.; Zhang, S.; Morris, M.E. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006, 8, E433–E442. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Wahajuddin, M.; Jain, G.K. Intravenous pharmacokinetics and oral bioavailability of biochanin A in female rats. Med. Chem. Res. 2011, 20, 1627–1631. [Google Scholar] [CrossRef]
- Srinivas, N.R. Biochanin A: Understanding the complexities in the paradoxical drug–drug interaction potential. Eur. J. Drug Metab. Pharmacokinetics 2015, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Lou, L.; Wang, Y. Perspectives on the Role of Biochanin A in human. Front. Pharmacol. 2019, 10, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Ge, W.; Shao, T.; Wu, W.; Hou, J.; Cui, L.; Wang, J.; Zhang, Z. Enhancing the oral bioavailability of biochanin A by encapsulation in mixed micelles containing Pluronic F127 and Plasdone S630. Int. J. Nanomed. 2017, 12, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, C.; Mishra, N.; Sharma, S. Development and characterization of enteric-coated microparticles of biochanin A for their beneficial pharmacological potential in estrogen deficient-hypertension. Drug Deliv. 2016, 23, 2044–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Cheng, H.; Zhou, K.; Luo, Q.; Guo, L.; Chen, W. Preparation and characterization of Biochanin A loaded solid lipid nanoparticles. Asian J. Pharm. 2012, 6, 275–281. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, H.; Zhou, K.; Wang, L.; Dong, S.; Wang, D.; Chen, W. Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 2013, 20, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Hanski, L.; Genina, N.; Uvell, H.; Malinovskaja, K.; Gylfe, Å.; Laaksonen, T.; Kolakovic, R.; Makila, E.; Salonen, J.; Hirvonen, J.; et al. Inhibitory activity of the isoflavone biochanin A on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation. PLoS ONE 2014, 9, e115115. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Chen, X.; Liu, X.; Wang, Z.; Li, Y. Recent developments in polydopamine fluorescent nanomaterials. Mater. Horiz. 2020, 7, 746–761. [Google Scholar] [CrossRef]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Ilić-Stojanović, S.S.; Nikolić, L.B.; Nikolić, V.D.; Milić, J.R.; Stamenković, J.; Nikolić, G.M.; Petrović, S.D. Synthesis and characterization of thermosensitive hydrogels and the investigation of modified release of ibuprofen. Hem. Ind. 2013, 67, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Peppas, N.A. Synthesis and characterization of pH-and temperature-sensitive poly(methacrylic acid)/poly(N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 2000, 33, 102–107. [Google Scholar] [CrossRef]
- Osada, Y.; Gong, Y.P. Soft and Wet Materials: Polymer Gels. Adv. Mater. 1998, 10, 827–837. [Google Scholar] [CrossRef]
- Mohsen, R.; Alexander, B.D.; Richardson, S.C.W.; Mitchell, J.C.; Diab, A.A.; Snowden, M.J. Design, synthesis, characterization and toxicity studies of Poly (N-Iso-Propylacrylamide-co-Lucifer Yellow) particles for drug delivery applications. J. Nanomed. Nanotechnol. 2016, 7, 363–372. [Google Scholar] [CrossRef]
- Lin, X.; Tang, D.; Yu, Z.; Feng, Q. Stimuli-responsive electrospun nanofibers from poly (N-isopropylacrylamide)-co-poly (acrylic acid) copolymer and polyurethane. J. Mater. Chem. B 2014, 2, 651–658. [Google Scholar] [CrossRef]
- Adimi, M.; Attar, H.; Barati, A.; Seifkordi, A.; Bakhtiari, M. Experimental investigation and modeling of the anti-cancer drug delivery from poly(N-isopropylacrylamide-co-Acrylic acid) copolymeric hydrogels. Int. J. Biosci. 2014, 5, 183–191. [Google Scholar] [CrossRef]
- Bajpai, S.K. Swelling–deswelling behavior of poly(acrylamide-co-maleic acid) hydrogels. J. Appl. Polym. Sci. 2001, 80, 2782–2789. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Hansen, C.M. The significance of the surface condition in solutions to the diffusion equation: Explaining “anomalous” sigmoidal, Case II, and Super Case II absorption behavior. Eur. Polym. J. 2010, 46, 651–662. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Lin, Z. Kinetics and thermodynamics of the water sorption of 2-hydroxyethyl methacrylate/styrene copolymer hydrogels. J. Appl. Polym. Sci. 2008, 109, 3018–3023. [Google Scholar] [CrossRef]
- Telford, J.K. A Brief Introduction to Design of Experiments. Johns Hopkins APL Tech. Dig. 2007, 27, 224–232. [Google Scholar]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, A.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemometr. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Naeem, H.; Farooqi, Z.H.; Shah, L.A.; Siddiq, M. Synthesis and characterization of p(NIPAM-AA-AAm) microgels for tuning of optical properties of silver nanoparticles. J. Polym. Res. 2012, 19, 9950. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Synthesis and characterization of multiresponsive core–shell microgels. Macromolecules. 2000, 33, 8301–8306. [Google Scholar] [CrossRef]
- Kim, J.; Serpe, M.J.; Lyon, L.A. Hydrogel microparticles as dynamically tunable microlenses. J. Am. Chem. Soc. 2004, 126, 9512–9513. [Google Scholar] [CrossRef]
- Umemura, J.; Hayashi, S. Infrared spectra and molecular configurations of liquid and crystalline acrylic acids. Bull. Inst. Chem. Res. Kyoto Univ. 1975, 52, 585–595. [Google Scholar]
- Farooqi, Z.H.; Sakhawat, T.; Khan, S.R.; Kanwal, F.; Usman, M.; Begum, R. Synthesis, characterization and fabrication of copper nanoparticles in N-isopropylacrylamide based co-polymer microgels for degradation of p-nitrophenol. Mater. Sci. Poland. 2015, 33, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Kostic, M.; Pejcic, A.; Igic, M.; Gligorijevic, N. Adverse reactions to denture resin materials. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5298–5305. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.H.; Khan, H.U.; Shah, S.M.; Siddiq, M. Stability of poly(N-isopropylacrylamide-co-acrylic acid) polymer microgels under various conditions of temperature, pH and salt concentration. Arab. J. Chem. 2017, 10, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Ilić-Stojanović, S.; Nikolić, L.; Zdravković, A.; Nikolić, V. Procedure for Synthesis of Superapsorbing Temperature and pH Sensitive Hydrogels. Registered Patent RS 59327 B1. RS Patent Application 2016P01134 A1, 31 October 2019. [Google Scholar]
- Zdravkovic, A.S.; Nikolić, L.B.; Ilić-Stojanović, S.S.; Nikolić, V.D.; Savić, S.R.; Kapor, A.J. The evaluation of temperature and pH influences on equilibrium swelling of poly(N-isopropylacrylamide-co-acrylic acid) hydrogels. Hem. Ind. 2017, 71, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Z.; Yang, Y.Y.; Wang, F.J.; Chung, T.S. Thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with expanded network structures and improved oscillating swelling–deswelling properties. Langmuir 2002, 18, 2013–2018. [Google Scholar] [CrossRef]
- Garba, Z.N.; Rahim, A.A.; Hamza, S.A. Potential of Borassus aethiopum shells as precursor for activated carbon preparation by physico-chemical activation; Optimization, equilibrium and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1423–1433. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Biodiesel production using alumina-supported calcium oxide: An optimization study. Fuel Process. Technol. 2010, 91, 243–248. [Google Scholar] [CrossRef]
- Chrzanowska, A.M.; Poliwoda, A.; Wieczorek, P.P. Characterization of particle morphology of biochanin A molecularly imprinted polymers and their properties as a potential sorbent for solid-phase extraction. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 793–798. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- O’Hanlon, D.; Moench, T.; Cone, R. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bitterman, W.; Spencer, R.J.; Huizenga, K.A.; Shorter, R.G. Contact pH of rectal mucosa in humans and dogs. Dis. Colon. Rectum. 1969, 12, 96–98. [Google Scholar] [CrossRef]















| Factors | Coded | Actual | Level Values | |
|---|---|---|---|---|
| Coded | Actual | |||
| Temperature (°C) | X1 | t | −1 0 1 | 25 31 37 |
| pH | X2 | pH | −1 0 1 | 3.5 6.0 8.5 |
| p(NIPAM-co-AA) Sample | NIPAM | AA | EGDM | |||
|---|---|---|---|---|---|---|
| mg/g | % | mg/g | % | mg/g | % | |
| 95/5/1.5 | 1.937 | 0.212 | 0.103 | 0.175 | 0.101 | 0.382 |
| Temperature (°C) | pH | Before Lyophilization | After Lyophilization | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | k × 102 (min1/n) | R2 | D × 107 (cm2/min) | n | k (min1/n) | R2 | D × 104 (cm2/min) | ||
| 25 | 3.5 | 1.014 | 1.420 | 0.989 | 3.960 | 0.190 | 0.295 | 0.977 | 1.700 |
| 25 | 8.5 | 0.844 | 1.716 | 0.972 | 5.778 | 0.303 | 0.222 | 0.990 | 0.972 |
| 37 | 3.5 | 0.822 | 1.567 | 0.975 | 4.820 | 0.282 | 0.243 | 0.981 | 1.160 |
| 37 | 8.5 | 0.936 | 0.503 | 0.995 | 0.496 | 0.222 | 0.286 | 0.976 | 1.610 |
| Number of Experiment | X1 t (°C) | X2 pH | Yexp. αe |
|---|---|---|---|
| 9 | 37 | 8.5 | 121.586 |
| 7 | 25 | 8.5 | 269.323 |
| 3 | 37 | 3.5 | 7.921 |
| 5 | 31 | 6.0 | 206.836 |
| 4 | 25 | 6.0 | 216.36 |
| 6 | 37 | 6.0 | 94.872 |
| 1 | 25 | 3.5 | 11.531 |
| 8 | 31 | 8.5 | 239.891 |
| 2 | 31 | 3.5 | 10.057 |
| Source | SS | df | MS | F | p | |
|---|---|---|---|---|---|---|
| Model | 87,752.58 | 5 | 17,550.52 | 30.65 | 0.0089 | significant |
| X1 | 12,406.49 | 1 | 12,406.49 | 21.67 | 0.0187 | |
| X2 | 60,258.48 | 1 | 60,258.48 | 105.25 | 0.0020 | |
| X1X2 | 5193.15 | 1 | 5193.15 | 9.07 | 0.0571 | |
| 2047.47 | 1 | 2047.47 | 3.58 | 0.1550 | ||
| 7847.00 | 1 | 7847.00 | 13.71 | 0.0342 | ||
| Residual | 1717.66 | 3 | 572.55 | |||
| Cor Total | 89,470.23 | 8 |
| Hydrogel Sample | Mass of Xerogel (g) | Lg (mg/gxerogel) | ηbiochanin A (%) |
|---|---|---|---|
| p(NIPAM-co-AA) non-lyophilized | 0.0206 | 55.660 | 92.767 |
| p(NIPAM-co-AA) lyophilized | 0.0196 | 58.323 | 97.205 |
| Temperature (°C) | pH | Non-Lyophilized | Lyophilized | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | k (min1/n) | R2 | D × 105 (cm2/min) | n | k (min1/n) | R2 | D × 104 (cm2/min) | ||
| 37 | 4.5 | 0.274 | 0.197 | 0.984 | 3.577 | 0.257 | 0.217 | 0.997 | 4.252 |
| 37 | 7.9 | 0.439 | 0.106 | 0.966 | 1.807 | 0.292 | 0.185 | 0.976 | 2.695 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajić, I.; Ilić-Stojanović, S.; Dinić, A.; Zdravković, A.; Stanojević, L.; Nikolić, V.; Nikolić, L. Modified Biochanin A Release from Dual pH- and Thermo-Responsive Copolymer Hydrogels. Polymers 2021, 13, 426. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030426
Gajić I, Ilić-Stojanović S, Dinić A, Zdravković A, Stanojević L, Nikolić V, Nikolić L. Modified Biochanin A Release from Dual pH- and Thermo-Responsive Copolymer Hydrogels. Polymers. 2021; 13(3):426. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030426
Chicago/Turabian StyleGajić, Ivana, Snežana Ilić-Stojanović, Ana Dinić, Aleksandar Zdravković, Ljiljana Stanojević, Vesna Nikolić, and Ljubiša Nikolić. 2021. "Modified Biochanin A Release from Dual pH- and Thermo-Responsive Copolymer Hydrogels" Polymers 13, no. 3: 426. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13030426