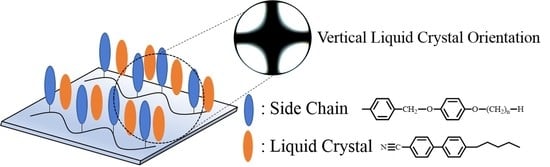
Vertical Orientation of Liquid Crystal on 4-n-Alkyloxyphenoxymethyl-Substituted Polystyrene Containing Liquid Crystal Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of 4-n-Alkyloxyphenoxymethyl Modified Polystyrene
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Chen, J.Z. General liquid-crystal theory for anisotropically shaped molecules: Symmetry, orientational order parameters, and system free energy. Phys. Rev. E 2020, 102, 062701. [Google Scholar] [CrossRef] [PubMed]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Oxford University Press: Oxford, UK, 1993; pp. 337–393. [Google Scholar]
- Alla, A.; Saeed, R.M. On the Control of Nematic Liquid Crystal Alignment. Ph.D. Thesis, University of Gothenburg, Göteborg, Sweden.
- Goujon, F.; Martzel, N.; Dequidt, A.; Latour, B.; Garruchet, S.; Devémy, J.; Blaak, R.; Munch, É.; Malfreyt, P. Backbone oriented anisotropic coarse grains for efficient simulations of polymers. J. Chem. Phys. 2020, 153, 214901. [Google Scholar] [CrossRef]
- Grasinger, M.; Dayal, K. Architected elastomer networks for optimal electromechanical response. J. Mech. Phys. Solids 2021, 146, 104171. [Google Scholar] [CrossRef]
- Castañeda, P.P.; Galipeau, E. Homogenization-based constitutive models for magnetorheological elastomers at finite strain. J. Mech. Phys. Solids 2011, 59, 194–215. [Google Scholar] [CrossRef]
- Galipeau, E.; Castañeda, P.P. A finite-strain constitutive model for magnetorheological elastomers: Magnetic torques and fiber rotations. J. Mech. Phys. Solids 2013, 61, 1065–1090. [Google Scholar] [CrossRef]
- Kurabayashi, K. Anisotropic thermal properties of solid polymers. Int. J. Thermophys. 2001, 22, 277–288. [Google Scholar] [CrossRef]
- Kurabayashi, K.; Asheghi, M.; Touzelbaev, M.; Goodson, K.E. Measurement of the thermal conductivity anisotropy in polyimide films. J. Microelectromech. Syst. 1999, 8, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Zhao, T.; Wang, M.; Deng, L.; Lin, B.; Zhang, X.; Sun, Y.; Yang, H.; Chen, E. A homeotropic main-chain tolane-type liquid crystal elastomer film exhibiting high anisotropic thermal conductivity. Soft Matter 2017, 13, 5463–5468. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.; Takezoe, H.; Haba, O.; Yonetake, K.; Morikawa, J. Photo-controllable thermal diffusivity and thermal conductivity driven by the orientation change of nematic liquid crystal with azo-dendrimers. Appl. Phys. Lett. 2015, 107, 221901. [Google Scholar] [CrossRef]
- Gupta, M.K.; Srivastava, R.K. Mechanical properties of hybrid fibers-reinforced polymer composite: A review. Polym.-Plast. Technol. Eng. 2016, 55, 626–642. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Liu, Y.; Cao, A.; Qian, W.; Lu, Y.; Wei, F. Energy-absorbing hybrid composites based on alternate carbon-nanotube and inorganic layers. Adv. Mater. 2009, 21, 2876–2880. [Google Scholar] [CrossRef]
- Shambina, S.L.; Virchenko, G.A. Special features of design and calculation for structures made of anisotropic fiberglass. IOP Conf. Ser. Mater. Sci. Eng. 2017, 222, 012011. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Szymoniak, P.; Sentker, K.; Butschies, M.; Bühlmeyer, A.; Huber, P.; Laschat, S.; Schönhals, A. Dynamics and ionic conductivity of ionic liquid crystals forming a hexagonal columnar mesophase. Phys. Chem. Chem. Phys. 2018, 20, 5626–5635. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Kamikawa, Y.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Ionic liquid crystals: Self-assembly of imidazolium salts containing an L-glutamic acid moiety. Chem. Lett. 2008, 37, 538–539. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Yamaguchi, A.; Alam, M.A.; Yamamoto, Y.; Fukushima, T.; Kato, K.; Takata, M.; Fujita, N.; Aida, T. Discotic ionic liquid crystals of triphenylene as dispersants for orienting single-walled carbon nanotubes. Angew. Chem. 2012, 124, 8618–8622. [Google Scholar] [CrossRef]
- Choy, C.L.; Wong, S.P.; Young, K. Model calculation of the thermal conductivity of polymer crystals. J. Polym. Sci. Pt. B-Polym. Phys. 1985, 23, 1495–1504. [Google Scholar] [CrossRef]
- Hong, S.M.; Kim, S.H.; Kim, J.H.; Hwang, H.I. Hydrophilic surface modification of PDMS using atmospheric RF plasma. J. Phys. Conf. Ser. 2006, 34, 656–661. [Google Scholar] [CrossRef]
- Meincken, M.; Berhane, T.A.; Mallon, P.E. Tracking the hydrophobicity recovery of PDMS compounds using the adhesive force determined by AFM force distance measurements. Polymer 2005, 46, 203–208. [Google Scholar] [CrossRef]
- Boxshall, K.; Wu, M.; Cui, Z.; Cui, Z.; Watts, J.F.; Baker, M.A. Simple surface treatments to modify protein adsorption and cell attachment properties within a poly(dimethylsiloxane) micro-bioreactor. Surf. Interface Anal. 2006, 38, 198–201. [Google Scholar] [CrossRef]
- Paguirigan, A.L.; Beebe, D.J. From the cellular perspective: Exploring differences in the cellular baseline in macroscale and microfluidic cultures. Integr. Biol. 2009, 1, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.; Whitesides, G.M. Method for fabrication of microfluidic systems in glass. Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A. Modification of polysiloxane polymers for biomedical applications: A review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Stohr, J.; Samant, M.G.; Cossy-Favre, A.; Daiz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar] [CrossRef]
- Ge, J.J.; Li, C.Y.; Xue, G.; Mann, I.K.; Zhang, D.; Wang, S.; Harris, F.W.; Cheng, S.Z.; Hong, S.; Zhuang, X. Rubbing-induced molecular reorientation on an alignment surface of an aromatic polyimide containing cyanobiphenyl side chains. J. Am. Chem. Soc. 2001, 123, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Takatoh, K.; Sakamoto, M.; Hasegawa, R.; Koden, M.; Itoh, N.; Hasegawa, M. Alignment Technology and Applications of Liquid Crystal Devices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J.; Samant, M.G. Liquid crystal alignment by rubbed polymer surfaces: A microscopic bond orientation model. J. Electron Spectrosc. Relat. Phenom. 1999, 98, 189–207. [Google Scholar] [CrossRef]
- Prasad, S.K.; Nair, G.G.; Hegde, G. Nonequilibrium liquid crystalline layered phase stabilized by light. J. Phys. Chem. B 2007, 111, 345–350. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Ghosh, M. Polyimides: Fundamentals and Applications, 1st ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 367–388. [Google Scholar]
- Van Aerle, N.A.J.M.; Tol, J.W. Molecular orientation in rubbed polyimide alignment layers used for liquid-crystal displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar] [CrossRef]
- Lee, K.W.; Paek, S.H.; Lien, A.; Durning, C.; Fukuro, H. Microscopic molecular reorientation of alignment layer polymer surfaces induced by rubbing and its effects on LC pretilt angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar] [CrossRef]
- Weiss, K.; Wöll, C.; Böhm, E.; Fiebranz, B.; Forstmann, G.; Peng, B.; Scheumann, V.; Johannsmann, D. Molecular orientation at rubbed polyimide surfaces determined with x-ray absorption spectroscopy: Relevance for liquid crystal alignment. Macromolecules 1998, 31, 1930–1936. [Google Scholar] [CrossRef]
- Meister, R.; Jerome, B. Configuration of a rubbed polymer. Macromolecules 1999, 32, 480–486. [Google Scholar] [CrossRef]
- Kim, D.; Oh–e, M.; Shen, Y.R. Rubbed polyimide surface studied by sum–frequency vibrational spectroscopy. Macromolecules 2001, 34, 9125–9129. [Google Scholar] [CrossRef]
- Vaughn, K.E.; Sousa, M.; Kang, D.; Rosenblatt, C. Continuous control of liquid crystal pretilt angle from homeotropic to planar. Appl. Phys. Lett. 2007, 90, 194102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Reea, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid crystal alignment on the rubbed film surface of semi–flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A-Mol. Cryst. Liq. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.W.; Ha, J.D.; Oh, J.M.; Yi, M.H. Synthesis and characterization of novel polyimides with 1-octadecyl side chains for liquid crystal alignment layers. Polym. Adv. Technol. 2007, 18, 226–234. [Google Scholar] [CrossRef]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I.; Park, S.; Jung, J.C.; Lee, K.H.; Ree, M. Rubbing–induced surface morphology and polymer segmental reorientations of a model brush polyimide and interactions with liquid crystals at the surface. Chem. Mat. 2003, 15, 3105–3112. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, G.J.; Chi, J.H.; Zin, W.; Jung, J.C.; Hahm, S.G.; Ree, M.; Chang, T. Synthesis, characterization and liquid-crystal-aligning properties of novel aromatic polypyromellitimides bearing (n-alkyloxy) biphenyloxy side chains. Polymer 2006, 47, 6606–6621. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J. Liquid crystal alignment property of n-alkylthiomethyl-or n-alkylsulfonylmethyl-substituted polystyrenes. Polym. Adv. Technol. 2009, 20, 878–886. [Google Scholar] [CrossRef]
- Hanemann, T.; Haase, W. Crystal structure of 4′-pentyl-4-cyanobiphenyl (5CB). Liq. Cryst. 1995, 19, 699–702. [Google Scholar] [CrossRef]
- Bogi, A.; Faetti, S. Elastic, dielectric and optical constants of 4′-pentyl-4-cyanobiphenyl. Liq. Cryst. 2001, 28, 729–739. [Google Scholar] [CrossRef]
- Maze, C. Determination of nematic liquid crystal elastic and dielectric properties from the shape of a capacitance-voltage curve. Mol. Cryst. Liq. Cryst. 1978, 48, 273–287. [Google Scholar] [CrossRef]
- Schell, K.T.; Porter, R.S. Dielectric studies of highly polar nematic liquid crystals and their mixtures. Liq. Cryst. 1990, 188, 97–103. [Google Scholar] [CrossRef]
- Owens, D.K. Some thermodynamic aspects of polymer adhesion. J. Appl. Polym. Sci. 1970, 14, 1725–1730. [Google Scholar] [CrossRef]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef]
- Royall, P.G.; Craig, D.Q.; Doherty, C. Characterisation of the glass transition of an amorphous drug using modulated DSC. Pharm. Res. 1998, 15, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J. Determination of the glass transition temperature: Methods correlation and structural heterogeneity. J. Therm. Anal. 2009, 98, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 5, 318–321. [Google Scholar] [CrossRef]
- Wesslen, B.; Lenz, R.W.; MacKnight, W.J.; Karasz, F.E. Glass transition temperatures of poly(ethyl α-chloroacrylates). Macromolecules 1971, 4, 24–26. [Google Scholar] [CrossRef]
- Lee, J.; Litt, M.H.; Rogers, C.E. Oxyalkylene polymers with alkylsulfonylmethyl side chains: Gas barrier properties. J. Polym. Sci. Pt. B-Polym. Phys. 1998, 36, 75–83. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 129–187. [Google Scholar]
- Senta, R.; Leo, M. Glass transitions of the poly-(n-alkyl methacrylates). J. Phys. Chem. 1957, 61, 985–991. [Google Scholar]
- Kahn, F.J.; Taylor, G.N.; Schonhorn, H. Surface-produced alignment of liquid crystals. Proc. IEEE 1973, 61, 823–828. [Google Scholar] [CrossRef]
- Kim, S.I.; Ree, M.; Shin, T.J.; Jung, J.C. Synthesis of new aromatic polyimides with various side chains containing a biphenyl mesogen unit and their abilities to control liquid-crystal alignments on the rubbed Surface. J. Polym. Sci. Pol. Chem. 1999, 37, 2909–2921. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Mendoza, A.M.; Wattanatorn, N.; Zhao, Y.; Nguyen, V.T.; Spokoyny, A.M.; Mirkin, C.A.; Baše, T.; Weiss, P.S. Surface dipole control of liquid crystal alignment. J. Am. Chem. Soc. 2016, 138, 5957–5967. [Google Scholar] [CrossRef] [Green Version]
- Bouchiat, M.A.; Langevin-Cruchon, D. Molecular order at the free surface of a nematic liquid crystal from light reflectivity measurements. Phys. Lett. A 1971, 34, 331–332. [Google Scholar] [CrossRef]
- Haller, I. Alignment and wetting properties of nematic liquids. Appl. Phys. Lett. 1974, 24, 349–351. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers1. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar] [CrossRef]
- Büchel, M.; Weichart, B.; Minx, C.; Menzel, H.; Johannsmann, D. Role of interfacial entropy in the command-surface effect. Phys. Rev. E 1997, 55, 455–463. [Google Scholar] [CrossRef]
- Creagh, L.T.; Kmetz, A.R. Mechanism of surface alignment in nematic liquid crystals. Mol. Cryst. Liq. Cryst. 1973, 24, 59–68. [Google Scholar] [CrossRef]







| Polymer Designation | Feed Ratio of 4-n-Alkyloxyphenol (mol%) | Degree of Substitution (mol%) | Tg (°C) |
|---|---|---|---|
| PEOP20 | 20 | 20 | 100.0 |
| PEOP40 | 40 | 40 | 81.7 |
| PEOP60 | 60 | 60 | 78.3 |
| PEOP80 | 80 | 80 | 74.0 |
| PEOP | 150 | 100 | 61.2 |
| PBOP | 150 | 100 | 47.3 |
| PHOP | 150 | 100 | 37.7 |
| POOP | 150 | 100 | 38.4 |
| Polymer Designation | Contact Angle (°) a | Surface Energy (mJ/m2) b | Vertical LC Aligning Ability | |||
|---|---|---|---|---|---|---|
| Water | Methylene Iodide | Dispersion | Polar | Total | ||
| PEOP20 | 90 | 23 | 46.1 | 0.6 | 46.7 | No |
| PEOP40 | 82 | 31 | 40.0 | 3.2 | 43.2 | Yes |
| PEOP60 | 82 | 31 | 40.0 | 3.2 | 43.2 | Yes |
| PEOP80 | 82 | 32 | 39.3 | 3.6 | 42.9 | Yes |
| PEOP | 83 | 33 | 39.4 | 3.0 | 42.4 | Yes |
| PBOP | 90 | 39 | 38.2 | 1.4 | 39.6 | Yes |
| PHOP | 92 | 52 | 30.7 | 2.0 | 32.7 | Yes |
| POOP | 92 | 77 | 15.1 | 5.9 | 21.0 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, K.; Kang, H. Vertical Orientation of Liquid Crystal on 4-n-Alkyloxyphenoxymethyl-Substituted Polystyrene Containing Liquid Crystal Precursor. Polymers 2021, 13, 736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050736
Seo K, Kang H. Vertical Orientation of Liquid Crystal on 4-n-Alkyloxyphenoxymethyl-Substituted Polystyrene Containing Liquid Crystal Precursor. Polymers. 2021; 13(5):736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050736
Chicago/Turabian StyleSeo, Kyutae, and Hyo Kang. 2021. "Vertical Orientation of Liquid Crystal on 4-n-Alkyloxyphenoxymethyl-Substituted Polystyrene Containing Liquid Crystal Precursor" Polymers 13, no. 5: 736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050736