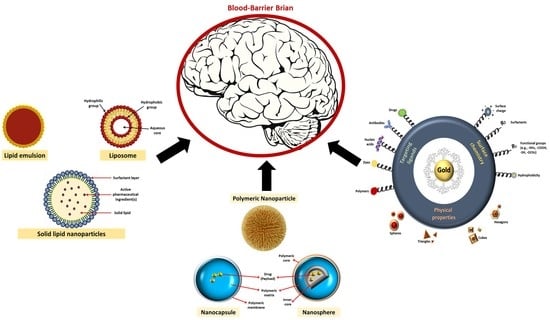
Potential Role of Nanoparticles in Treating the Accumulation of Amyloid-Beta Peptide in Alzheimer’s Patients
Abstract
:1. Introduction
2. Polymeric Nanoparticles Effects on Amyloid Beta Peptide
2.1. Role of PLGA in Treating AD as a Polymeric Material
Combining Quercetin and PLGA as a Polymeric Material
2.2. Role of Pittsburgh Compound B (PiB) in Treating AD
3. Lipid Nanoparticles Effects on Amyloid Beta Peptide
3.1. Role of Curcumin in Overcoming Aβ
3.2. Role of Solid Lipids Nanoparticles (SLNs) in Treating AD
3.3. Merging Nanostructured Lipid Carriers (NLCs) and SLNs for Treating AD
4. Inorganic Materials for Overcoming Aβ
4.1. Gold Nanoparticles Effects on Amyloid Beta Peptide
4.2. Silver Effects on Amyloid Beta Peptide
4.3. Selenium Effects on Amyloid Beta Peptide
4.4. Iron Effects on Amyloid Beta Peptide
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca-Santos, B.; Gremião, M.P.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yang, J.; Liu, L.; Lu, Z.; Shi, Z.; Ji, W.; Shen, J.; Zhang, X. An “Amyloid-β Cleaner” for the Treatment of Alzheimer’s Disease by Normalizing Microglial Dysfunction. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Fatima, M.; Islam, Z.; Khan, R.; Uversky, V.; Salahuddin, P. Nanoparticle formulations in the diagnosis and therapy of Alzheimer’s disease. Int. J. Biol. Macromol. 2019, 130, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Dao, P.; Chen, H.; Yan, L.; Li, Y.; Zhang, W.; Li, L.; Du, Z.; Dong, C.; Meunier, B. Ultrasmall superparamagnetic iron oxide nanoparticles bound NIR dyes: Novel theranostic agents for Alzheimer’s disease. Dye. Pigment. 2020, 173, 107968. [Google Scholar] [CrossRef]
- Luo, S.; Ma, C.; Zhu, M.; Ju, W.; Yang, Y.; Wang, X. Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases With Emphasis on Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [Green Version]
- Ordóñez-Gutiérrez, L.; Wandosell, F. Nanoliposomes as a Therapeutic Tool for Alzheimer’s Disease. Front. Synaptic Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Maher, B.A. Airborne Magnetite- and Iron-Rich Pollution Nanoparticles: Potential Neurotoxicants and Environmental Risk Factors for Neurodegenerative Disease, Including Alzheimer’s Disease. J. Alzheimer’s Dis. Jad 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Mu, W.; Wei, D.; Zhang, Y.; Duan, Y.; Gao, J.; Gong, X.; Wang, H.; Wu, X.; Tao, H.; et al. A Novel Targeted and High-Efficiency Nanosystem for Combinational Therapy for Alzheimer’s Disease. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Bahadur, S.; Sachan, N.; Harwansh, R.K.; Deshmukh, R. Nanoparticlized System: Promising Approach for the Management of Alzheimer’s Disease through Intranasal Delivery. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Verma, R.K.; Chawla, V.; Bhattamisra, S.; Gorain, B.; Raja, M.A.; Amjad, M. Nanoparticles Based Intranasal Delivery of Drug to Treat Alzheimer’s Disease: A Recent Update. CNS Neurol. Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- Liu, D.; Fu, D.; Zhang, L.; Sun, L. Detection of amyloid-beta by Fmoc-KLVFF self-assembled fluorescent nanoparticles for Alzheimer’s disease diagnosis. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; Pereira, M.C. Nanomaterials towards Biosensing of Alzheimer’s Disease Biomarkers. Nanomaterials 2019, 9, 1663. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; An, S.; Li, J.; Kuang, Y.; He, X.; Guo, Y.; Ma, H.; Zhang, Y.; Ji, B.; Jiang, C. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 2016, 80, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, S.; Yang, L.; Wang, X.; Qi, C.; Yang, R.; Wang, C. Molybdenum Disulfide Nanoparticles as Multifunctional Inhibitors against Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2017, 9, 21116–21123. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.M.; Rijo, P.; Reis, C. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef]
- Moore, K.A.; Pate, K.M.; Soto-Ortega, D.D.; Lohse, S.E.; Munnik, N.P.; Lim, M.; Jackson, K.S.; Lyles, V.D.; Jones, L.; Glassgow, N.; et al. Influence of gold nanoparticle surface chemistry and diameter upon Alzheimer’s disease amyloid-β protein aggregation. J. Biol. Eng. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.; Kim, M.; Réhman, S.U.; Ahmad, A. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2016, 54, 6490–6506. [Google Scholar] [CrossRef]
- Morales-Zavala, F.; Arriagada, H.; Hassan, N.; Velasco, C.; Riveros, A.; Álvarez, A.R.; Minniti, A.N.; Rojas-Silva, X.; Muñoz, L.; Vásquez, R.; et al. Peptide multifunctionalized gold nanorods decrease toxicity of β-amyloid peptide in a Caenorhabditis elegans model of Alzheimer’s disease. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2341–2350. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chemistry 2015, 2, 829–835. [Google Scholar] [CrossRef]
- Yao, L.; Gu, X.; Song, Q.; Wang, X.; Huang, M.; Hu, M.; Hou, L.; Kang, T.; Chen, J.; Chen, H.; et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J. Control. Release Off. J. Control. Release Soc. 2016, 226, 1–14. [Google Scholar] [CrossRef]
- Liu, C.; Lu, D.; You, X.; Shi, G.; Deng, J.; Zhou, T. Carbon dots sensitized lanthanide infinite coordination polymer nanoparticles: Towards ratiometric fluorescent sensing of cerebrospinal Aβ monomer as a biomarker for Alzheimer’s disease. Anal. Chim. Acta 2020, 1105, 147–154. [Google Scholar] [CrossRef]
- Pinheiro, R.; Granja, A.; Loureiro, J.; Pereira, M.C.; Pinheiro, M.; Neves, A.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-Capped Metal Nanoparticles Inhibit Tau Aggregation in Alzheimer’s Disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.; Wáng, Y.J.; Chow, A.H.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.; Kim, S.; Koh, S.; Han, S.; Yoon, M. Ultrasensitive Fluorescence Detection of Alzheimer’s Disease Based on Polyvalent Directed Peptide Polymer Coupled to a Nanoporous ZnO Nanoplatform. Anal. Chem. 2019, 91, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lim, E.; Fields, T.; Wu, H.; Xu, Y.; Wang, Y.A.; Mao, H. Improving Sensitivity and Specificity of Amyloid-β Peptides and Tau Protein Detection with Antibiofouling Magnetic Nanoparticles for Liquid Biopsy of Alzheimer’s Disease. ACS Biomater. Sci. Eng. 2019, 5, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Droumaguet, B.L.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordóñez-Gutiérrez, L.; et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 609–618. [Google Scholar] [CrossRef]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-targeted TPP-MoS2 with dual enzyme activity provides efficient neuroprotection through M1/M2 microglial polarization in an Alzheimer’s disease model. Biomaterials 2019, 232, 119752. [Google Scholar] [CrossRef] [PubMed]
- Aalinkeel, R.; Kutscher, H.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.; Prasad, P.N.; Mahajan, S. Neuroprotective effects of a biodegradable poly(lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: A potential nanotherapy for Alzheimer’s disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lu, S.; Liu, X.; Zhu, J.; Wang, Y.; Liu, R. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Abreu, M.; Calpena, A.; Andrés-Benito, P.; Asó, E.; Romero, I.; Roig-Carles, D.; Gromnicova, R.; Espina, M.; Ferrer, I.; García, M.; et al. PPARγ agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer’s disease: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 5577–5590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.; Goli, D. Formulation Preparation, Characterization, Optimization, Behavior and Histological Evaluation of Brain Hippocampus for Brain Targeted PLGA-Soya Lecithin-Tween 80 Nanoparticles in an Alzheimer’s Disease Model. Der Pharm Lett 2016, 8, 102–120. [Google Scholar]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef]
- Jeon, S.G.; Cha, M.; Kim, J.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.; Kim, J.; Moon, M. Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 297–307. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.; Espina, M.; Cano, A.; Calpena, A.; Camins, A.; Carmona, N.; Silva, A.; Souto, E.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.; Granja, A.; Loureiro, J.; Pereira, M.D.; Pinheiro, M.; Neves, A.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Wu, J.; Li, M.; Wang, P. A Novel Magnetic Nanoparticle for Early Detection of Amyloid Plaques in Alzheimer’s Disease. Arch. Med Res. 2018, 49, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, J.; Li, M.; Wang, P. In vitro early detection of amyloid plaques in Alzheimer’s disease by Pittsburgh compound B-modified magnetic nanoparticles. Zhonghua Yi Xue Za Zhi 2017, 97, 3258–3262. [Google Scholar]
- Selvan, S.T.; Ravichandar, R.; Ghosh, K.K.; Mohan, A.; Mahalakshmi, P.; Gulyás, B.; Padmanabhan, P. Coordination chemistry of ligands: Insights into the design of amyloid beta/tau-PET imaging probes and nanoparticles-based therapies for Alzheimer’s disease. Coord. Chem. Rev. 2020, 430, 213659. [Google Scholar] [CrossRef]
- López, E.H.; Machado, A.L.; Vidal, L.B.; Pizarro, R.G.; Silva, A.M.; Souto, E.B. Lipid nanoparticles as carriers for the treatment of neurodegeneration associated with Alzheimer’s disease and glaucoma: Present and future challenges. Curr. Pharm. Des. 2020, 26, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Posadino, A.M.; Pintus, G.; Sarmento, B.; Giunchedi, P.; Gavini, E. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf. B Biointerfaces 2017, 152, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.A.; Hashad, A.M.; Abdel-Reheem, A.Y. Formulation and evaluation of novel brain targeting drug loaded in lipid-based nanoparticles through intranasal route for alzheimer. Int. Res. J. Pharm. 2019, 10, 21–27. [Google Scholar] [CrossRef]
- Sathya, S.; Shanmuganathan, B.; Devi, K. Deciphering the anti-apoptotic potential of α-bisabolol loaded solid lipid nanoparticles against Aβ induced neurotoxicity in Neuro-2a cells. Colloids Surf. B Biointerfaces 2020, 190, 110948. [Google Scholar] [CrossRef]
- Topal, G.; Mészáros, M.; Porkoláb, G.; Szecskó, A.; Polgár, T.; Siklós, L.; Deli, M.; Veszelka, S.; Bozkır, A. ApoE-Targeting Increases the Transfer of Solid Lipid Nanoparticles with Donepezil Cargo across a Culture Model of the Blood–Brain Barrier. Pharmaceutics 2020, 13, 38. [Google Scholar] [CrossRef]
- Omar, S.H.; Osman, R.; Mamdouh, W.; Abdel-Bar, H.M.; Awad, G. Bioinspired lipid-polysaccharide modified hybrid nanoparticles as a brain-targeted highly loaded carrier for a hydrophilic drug. Int. J. Biol. Macromol. 2020, 165 Pt A, 483–494. [Google Scholar] [CrossRef]
- Fonseca-Gomes, J.; Loureiro, J.; Tanqueiro, S.R.; Mouro, F.M.; Ruivo, P.; Carvalho, T.; Sebastião, A.M.; Diógenes, M.J.; Pereira, M.C. In vivo Bio-Distribution and Toxicity Evaluation of Polymeric and Lipid-Based Nanoparticles: A Potential Approach for Chronic Diseases Treatment. Int. J. Nanomed. 2020, 15, 8609–8621. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Brain targeting of resveratrol through intranasal lipid vesicles labelled with gold nanoparticles: In vivo evaluation and bioaccumulation investigation using computed tomography and histopathological examination. J. Drug Target. 2019, 27, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Poka, L.P.; Mohan, G.; Rao, K.; Shanker, K. Neuroprotective Effect of Green Synthesized Iron Oxide Nanoparticles Using Aqueous Extract of Convolvulus Pluricaulis Plant in The Management of Alzheimer’s Disease. Phytopathology 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Ishak, R.A.; Mostafa, N.; Kamel, A.O. Stealth lipid polymer hybrid nanoparticles loaded with rutin for effective brain delivery—comparative study with the gold standard (Tween 80): Optimization, characterization and biodistribution. Drug Deliv. 2017, 24, 1874–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadpanah, M.; Dargahi, L.; Ai, J.; Taei, A.A.; Barough, S.E.; Mowla, S.J.; Tavoosidana, G.; Farahmandfar, M. Extracellular Vesicles as a Neprilysin Delivery System Memory Improvement in Alzheimer’s Disease. Iran. J. Pharm. Res. IJPR 2020, 19, 45–60. [Google Scholar] [PubMed]
- Harakeh, S.; Qari, M.; Ramadan, W.S.; Jaouni, S.K.; Muhayawi, M.A.; Amri, T.A.; Ashraf, G.M.; Bharali, D.J.; Mousa, S. Novel Nano-formulations of Ellagic Acid are Promising in Restoring Oxidative Homeostasis in Rat Brains with Alzheimer’s Disease. Curr. Drug Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sannikova, N.; Law, M.; Olaizola, A.; Lin, M.; Skrinskas, T. Impact of Lipid Composition on Liposome Stability and Cannabinoid Drug Encapsulation Efficiency. In ASI Nanomedicine Day, The NanoMedicines Innovation Network (NMIN); AScension Sciences Inc.: Vancouver, BC, Canada, 2020. [Google Scholar]
- Sintov, A. AmyloLipid Nanovesicles: A Self-Assembled Lipid-Modified Starch Hybrid System Constructed for Direct Nose-to-Brain Delivery of Curcumin. Int. J. Pharm. 2020, 588, 119725. [Google Scholar] [CrossRef]
- Nakama, K.A.; Santos, R.B.; Silva, C.E.; Izoton, J.C.; Savall, A.S.; Gutirrez, M.; Roman, S.; Luchese, C.; Pinton, S.; Haas, S. Establishment of analytical method for quantification of anti-inflammatory agents co-nanoencapsulated and its application to physicochemical development and characterization of lipid-core nanocapsules. Arab. J. Chem. 2020, 13, 2456–2469. [Google Scholar] [CrossRef]
- Ayaz, M.; Ovais, M.; Ahmad, I.; Sadiq, A.; Khalil, A.T.; Ullah, F. Chapter 3—Biosynthesized metal nanoparticles as potential Alzheimer’s disease therapeutics. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–42. [Google Scholar] [CrossRef]
- Singh, A.; Mahajan, S.; Kutscher, H.; Kim, S.; Prasad, P.N. Curcumin-Pluronic Nanoparticles: A Theranostic Nanoformulation for Alzheimer’s Disease. Crit. Rev. Biomed. Eng. 2020, 48, 153–168. [Google Scholar] [CrossRef]
- Kakkar, V.; Kumari, P.; Adlakha, S.; Kaur, I.P. Curcumin and Its Nanoformulations as Therapeutic for Alzheimer’s Disease. In Nanobiotechnology in Neurodegenerative Diseases; Springer: Cham, Switzerland, 2019; pp. 343–367. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Amini, A.; Javar, H.A.; Zarandi, B.F.; Montaseri, H.; Dinarvand, R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. Daru J. Pharm. Sci. 2018, 26, 165–177. [Google Scholar] [CrossRef]
- Loureiro, J.; Andrade, S.; Duarte, A.; Neves, A.; Queiroz, J.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.; et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Vedagiri, A.; Thangarajan, S. Mitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid β25–35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for Alzheimer’s disease. Neuropeptides 2016, 58, 111–125. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Dara, T.; Vatanara, A.; Sharifzadeh, M.; Khani, S.; Mosaddegh, M.H. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 1407082. [Google Scholar] [CrossRef]
- Kumar, R.; Garg, R.; Khurana, N. A comparative in vivo evaluation of anti-alzheimer activity of bacopa extract and its solid lipid nanoparticles. Curr. Bioact. Compd. 2020, 16. [Google Scholar] [CrossRef]
- Ravi, G.; Gupta, N.V. Development of Solid Lipid Nanoparticles of Rivastigmine Tartrate by Using Full Factorial Design for the Treatment of Alzheimer’s Disease. J. Pharm. Sci. Res. 2017, 9, 2447–2452. [Google Scholar]
- Malekpour-galogahi, F.; Hatamian-Zarmi, A.; Ganji, F.; Ebrahimi-Hosseinzadeh, B.; Nojoki, F.; Sahraeian, R.; Mokhtari-Hosseini, Z.B. Preparation and optimization of rivastigmine-loaded tocopherol succinate-based solid lipid nanoparticles. J. Liposome Res. 2018, 28, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Salve, P.; Pise, S.; Bali, N. Formulation and Evaluation of Solid Lipid Nanoparticle Based Transdermal Drug Delivery System for Alzheimer’s Disease. Res. J. Pharm. Pharm. Dos. Forms Technol. 2016, 8, 73–80. [Google Scholar] [CrossRef]
- Han, Y.; Gao, C.; Wang, H.; Sun, J.; Liang, M.; Feng, Y.; Liu, Q.; Fu, S.; Cui, L.; Gao, C.; et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact. Mater. 2021, 6, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Marto, J.; Gonçalves, L.; Almeida, A.; Vale, N. Formulation, Characterization and Evaluation against SH-SY5Y Cells of New Tacrine and Tacrine-MAP Loaded with Lipid Nanoparticles. Nanomaterials 2020, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Lopes, C.; Amaral, M.H.; Costa, P. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Suga, K.; Lai, Y.; Faried, M.; Umakoshi, H. Direct Observation of Amyloid β Behavior at Phospholipid Membrane Constructed on Gold Nanoparticles. Int. J. Anal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, P.; Loureiro, J.; Delerue-Matos, C.; Morais, S.; Pereira, M.D. Alzheimer’s disease: Development of a sensitive label-free electrochemical immunosensor for detection of amyloid beta peptide. Sens. Actuators B Chem. 2017, 239, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, F.; Hormozi-Nezhad, M.; Mahmoudi, M. Label-free detection of β-amyloid peptides (Aβ40 and Aβ42): A colorimetric sensor array for plasma monitoring of Alzheimer’s disease. Nanoscale 2018, 10, 6361–6368. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Wang, J.; Wang, Z.; Yi, X.; Wang, N. Voltammetric determination of the Alzheimer’s disease-related ApoE 4 gene from unamplified genomic DNA extracts by ferrocene-capped gold nanoparticles. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef]
- Lee, D.; Lee, G.; Yoon, D.S. Anti-Aβ drug candidates in clinical trials and plasmonic nanoparticle-based drug-screen for Alzheimer’s disease. Analyst 2018, 143, 2204–2212. [Google Scholar] [CrossRef]
- Hajipour, M.; Ghasemi, F.; Aghaverdi, H.; Raoufi, M.; Linne, U.; Atyabi, F.; Nabipour, I.; Azhdarzadeh, M.; Derakhshankhah, H.; Lotfabadi, A.; et al. Sensing of Alzheimer’s Disease and Multiple Sclerosis Using Nano-Bio Interfaces. J. Alzheimer’s Dis. JAD 2017, 59, 1187–1202. [Google Scholar] [CrossRef]
- Escosura-Muñiz, A.D.; Plichta, Z.; Horák, D.; Merkoci, A. Alzheimer’s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens. Bioelectron. 2015, 67, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, Y.; Liu, Q.; Gu, H.; Zhu, A.; Shi, G. Interface engineering of microelectrodes toward ultrasensitive monitoring of β-amyloid peptides in cerebrospinal fluid in Alzheimer’s disease. Analyst 2020. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, A.; Peng, Z.; Khan, A.S.; Junaid, M.; Ali, A.; Bharadwaj, S.; Wei, D. Evaluation and validation of synergistic effects of amyloid-beta inhibitor–gold nanoparticles complex on Alzheimer’s disease using deep neural network approach. J. Mater. Res. 2019, 34, 1845–1853. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.U.; Kim, S.; Song, S.; Sim, S. A Nanoplasmonic Biosensor for Ultrasensitive Detection of Alzheimer’s Disease Biomarker Using a Chaotropic Agent. ACS Sens. 2019, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, M.N.; Sezgintürk, M.K. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: Towards Alzheimer’s disease detection. Talanta 2020, 219, 121257. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Ferré-Borrull, J.; Kapruwan, P.; Marsal, L. A photoelectrochemical sandwich immunoassay for protein S100β, a biomarker for Alzheimer’s disease, using an ITO electrode modified with a reduced graphene oxide-gold conjugate and CdS-labeled secondary antibody. Microchim. Acta 2019, 186, 1–9. [Google Scholar] [CrossRef]
- Tramontin, N.D.; Silva, S.D.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; Silveira, G.D.; Mendes, C.; Silveira, P.C.; Muller, A.P. Gold Nanoparticles Treatment Reverses Brain Damage in Alzheimer’s Disease Model. Mol. Neurobiol. 2019, 57, 926–936. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, C.; Yan, F.; Sheng, Z. Synthesis of Chiral Penicillamine-Coated Gold Nanoparticles and Effect on PC12 Cells for the Treatment of Alzheimer’s Disease. J. Clust. Sci. 2019, 31, 1071–1075. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.; Pires, A.J.; Silveira, G.D.; Souza, D.L.; Brandolfi, J.D.; Souza, C.D.; Paula, M.M.; Silveira, P.C. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Kim, G.; Park, D.; Kim, J.; Kim, Y.S.; Kim, H.; Yang, S.; Lee, J.; Hwang, K.S. Gold nanoparticles assisted sensitivity improvement of interdigitated microelectrodes biosensor for amyloid-β detection in plasma sample. Sens. Actuators B Chem. 2020, 308, 127710. [Google Scholar] [CrossRef]
- Cendrowska, U.; Silva, P.J.; Ait-Bouziad, N.; Mueller, M.; Guven, Z.P.; Vieweg, S.; Chiki, A.; Radamaker, L.; Kumar, S.T.; Fändrich, M.; et al. Unraveling the complexity of amyloid polymorphism using gold nanoparticles and cryo-EM. Proc. Natl. Acad. Sci. USA 2020, 117, 6866–6874. [Google Scholar] [CrossRef] [Green Version]
- Jara-Guajardo, P.; Cabrera, P.; Celis, F.; Soler, M.; Berlanga, I.; Parra-Muñoz, N.; Acosta, G.A.; Albericio, F.; Guzmán, F.; Campos, M.; et al. Gold Nanoparticles Mediate Improved Detection of β-amyloid Aggregates by Fluorescence. Nanomaterials 2020, 10, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Córneo, E.D.; Silveira, G.D.; Scussel, R.; Corrêa, M.E.; Abel, J.D.; Luiz, G.P.; Feuser, P.E.; Silveira, P.; Machado-de-Ávila, R. Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of Parkinson’s disease. Colloids Surf. B Biointerfaces 2020, 196, 111302. [Google Scholar] [CrossRef]
- Talebpour, F.; Ghahghaei, A. Effect of Green Synthesis of Gold Nanoparticles (AuNPs) from Hibiscus sabdariffa on the Aggregation of α-Lactalbumin. Int. J. Pept. Res. 2020. [Google Scholar] [CrossRef]
- Karmakar, S.; Sarkar, N.; Pandey, L. Proline functionalized gold nanoparticles modulates lysozyme fibrillation. Colloids Surf. B Biointerfaces 2019, 174, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, M.; Gong, D.; Chen, R.; Hu, X.; Sun, T. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 2017, 9, 4107–4113. [Google Scholar] [CrossRef]
- Kim, M.; Réhman, S.U.; Amin, F.U. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-KB/JNK/GSK3β signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2533–2544. [Google Scholar] [CrossRef]
- Hu, T.; Lu, S.; Chen, C.; Sun, J.; Yang, X. Colorimetric sandwich immunosensor for Aβ(1–42) based on dual antibody-modified gold nanoparticles. Sens. Actuators B Chem. 2017, 243, 792–799. [Google Scholar] [CrossRef]
- Ruff, J.; Huewel, S.; Kogan, M.; Simon, U.; Galla, H. The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood-brain barrier. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1645–1652. [Google Scholar] [CrossRef]
- Das, T.; Kolli, V.; Karmakar, S.; Sarkar, N. Functionalisation of Polyvinylpyrrolidone on Gold Nanoparticles Enhances Its Anti-Amyloidogenic Propensity towards Hen Egg White Lysozyme. Biomedicines 2017, 5, 19. [Google Scholar]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Elbassal, E.A.; Morris, C.; Kent, T.W.; Lantz, R.L.; Ojha, B.; Wojcikiewicz, E.P.; Du, D. Gold Nanoparticles as a Probe for Amyloid-β Oligomer and Amyloid Formation. J. Phys. Chem. C Nanomater. Interfaces 2017, 121, 20007–20015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafuente, J.; Menon, P.; Moessler, H.; Muresanu, D.; Nozari, A.; Ozkizilcik, A.; Patnaik, R.; Sharma, A.; Sharma, H.; Tian, Z.R. Nanodelivery of Cerebrolysin Reduces Functionalized Gold Nanoparticles Induced Blood-Brain Barrier Disruption, Brain Edema Formation and Brain Pathology. TechConnect Briefs 2017, 3, 48–51. [Google Scholar]
- Delkhahi, S.; Rahaie, M.; Rahimi, F. Design and Fabrication a Gold Nanoparticle-DNA Based Nanobiosensor for Detection of microRNA Involved in Alzheimer’s Disease. J. Fluoresc. 2016, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Park, S. High Performance Detection of Alzheimer’s Disease Biomarkers Based on Localized Surface Plasmon Resonance. J. Ind. Eng. Chem. 2020, 91, 182–190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Hu, Y. Green synthesis of silver nanoparticles and their preventive effect in deficits in recognition and spatial memory in sporadic Alzheimer’s rat model. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125288. [Google Scholar] [CrossRef]
- Youssif, K.A.; Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Seleem, A.; Salem, M.; Hussein, A.A.; Krischke, M.; Mueller, M.; et al. Anti-Alzheimer potential, metabolomic profiling and molecular docking of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea aqueous extracts. PLoS ONE 2019, 14, e0223781. [Google Scholar] [CrossRef]
- Sikorska, K.; Gradzka, I.; Sochanowicz, B.; Presz, A.; Męczyńska-Wielgosz, S.; Brzóska, K.; Kruszewski, M. Diminished amyloid-β uptake by mouse microglia upon treatment with quantum dots, silver or cerium oxide nanoparticles: Nanoparticles and amyloid-β uptake by microglia. Hum. Exp. Toxicol. 2019, 39, 147–158. [Google Scholar] [CrossRef]
- Dehvari, M.; Ghahghaei, A. The effect of green synthesis silver nanoparticles (AgNPs) from Pulicaria undulata on the amyloid formation in α-lactalbumin and the chaperon action of α-casein. Int. J. Biol. Macromol. 2018, 108, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yin, Q.; Wang, J.; Yang, J.; Chen, Z.; Gao, Z.; Huang, Q.; Li, S. SERS-Based Immunoassay Enhanced with Silver Probe for Selective Separation and Detection of Alzheimer’s Disease Biomarkers. Int. J. Nanomed. 2021, 16, 1901–1911. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, H. Electrochemical sandwich-type biosensors for α-1 antitrypsin with carbon nanotubes and alkaline phosphatase labeled antibody-silver nanoparticles. Biosens. Bioelectron. 2017, 89 Pt 2, 959–963. [Google Scholar] [CrossRef]
- Lin, H.; Ho, M.; Tsen, C.; Huang, C.; Wu, C.; Huang, Y.; Hsiao, I.; Chuang, C. From the Cover: Comparative Proteomics Reveals Silver Nanoparticles Alter Fatty Acid Metabolism and Amyloid Beta Clearance for Neuronal Apoptosis in a Triple Cell Coculture Model of the Blood–Brain Barrier. Toxicol. Sci. 2017, 158, 151–163. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, J.; Liu, P.; Ji, D.; Zhang, L.; Zhang, M.; Li, Y.; Xiao, Y. Preparation and in vitro evaluation of multi-target-directed selenium-chondroitin sulfate nanoparticles in protecting against the Alzheimer’s disease. Int. J. Biol. Macromol. 2019, 142, 265–276. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Xie, W.; Liu, Y.; Liu, J. Dual-functional selenium nanoparticles bind to and inhibit amyloid β fiber formation in Alzheimer’s disease. J. Mater. Chem. B 2017, 5, 5954–5967. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, W.; Yu, Q.; Chen, X.; Xu, M.; Zhou, Y.; Liu, J. Chiral penicillamine-modified selenium nanoparticles enantioselectively inhibit metal-induced amyloid β aggregation for treating Alzheimer’s disease. J. Colloid Interface Sci. 2017, 505, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with B6 peptide for potential use in Alzheimer’s disease. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 575. [Google Scholar] [CrossRef]
- Vicente-zurdo, D.; Romero-Sánchez, I.; Rosales-Conrado, N.; Leon-gonzalez, M.; Madrid, Y. Ability of selenium species to inhibit metal-induced Aβ aggregation involved in the development of Alzheimer’s disease. Anal. Bioanal. Chem. 2020, 412, 6485–6497. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. Part A 2018, 106, 3034–3041. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Lu, S.; Liu, D.; Zhang, L.; Yu, X.; Liu, R. Multifunctional Superparamagnetic Iron Oxide Nanoparticles Conjugated with Aβ Oligomer-Specific scFv Antibody and Class A Scavenger Receptor Activator Show Early Diagnostic Potentials for Alzheimer’s Disease. Int. J. Nanomed. 2020, 15, 4919–4932. [Google Scholar] [CrossRef]
- Jajin, E.A.; Esmaeili, A.; Rahgozar, S.; Noorbakhshnia, M. Quercetin-Conjugated Superparamagnetic Iron Oxide Nanoparticles Protect AlCl3-Induced Neurotoxicity in a Rat Model of Alzheimer’s Disease via Antioxidant Genes, APP Gene, and miRNA-101. Front. Neurosci. 2020, 14, 598617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Lu, S.; Liu, D.; Huang, Y.; Zhu, J.; Zhou, W.; Yu, X.; Liu, R. Superparamagnetic iron oxide nanoparticles conjugated with Aβ oligomer-specific scFv antibody and class A scavenger receptor activator show therapeutic potentials for Alzheimer’s Disease. J. Nanobiotechnol. 2020, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Liu, J. Curcumin-Loaded Dual-Targets Nanoparticles with Enhanced Magnetic Resonance Imaging Therapy of Alzheimer’s disease in Transgenic Mice. J. Psychiatry 2020. [Google Scholar] [CrossRef]







| Classification | Name | Function and Merits | Average Diameter in nm | References |
|---|---|---|---|---|
| Polymeric nanoparticles | DGLs-PEG-RVG29-D-peptide/DNA NPs | It provides numerous reaction locations and good drug loading charges. Successful codelivery of blood-brain barrier-crossing drugs through brain-oriented ligand modifications was shown in vitro and in vivo. | 97 | [14] |
| Drug-loaded PLGA nanoparticles | In transgenic AD mice, spatial memory and recognition were significantly improved | 128.6 | [32] | |
| PLGA@QT NPs | Low cytotoxicity when tested in vitro on SH-SY5Y cells | Between 100 and 150 | [40] | |
| PGZ-NPs | The carriers received PGZ, which promoted 50 × higher brain endothelium absorption than free drugs and displayed a delayed in vitro release profile of PGZ. | 155.0 ± 1.8 | [33] | |
| Anti-A mAb-conjugated liposomes | Stable in serum protein incubation and in a position to bind to A in vitro monomers and fibrils | Between 124 and 134 | [16] | |
| Poly(acrylic acid)-coated NPs | Abrogated Aβ aggregation at a sub-stoichiometric ratio of 1:2,000,000 | 8 and 18 | [17] | |
| − PLGA NPs − PLGA NPs with OX26 mAb − PLGA NPs with OX26 mAb and DE2B4 mAb | The intake of immune nanoparticles with controlled peptide iA5 delivery without monoclonal antibody function was significantly increased. | 153 ± 2 163 ± 3 166 ± 2 | [35] | |
| PiB-MZF | It is stable, biocompatible. The relaxing rate of PiB-R2 MZF was 169.93 mM−1S−1, which showed great superparamagnetism as the negative T2 contrast agent. PiB-MZF also showed no cytotoxicity in two cell lines. | 100 | [43] | |
| NP(α−M) | Improving brain clearance in an LDLR-dependent way of 125I-radiolabeled Aβ1–42, reduction in Aβ deposition and reduced neuroinflammatory reactions. | 94.26 ± 4.54 | [20] | |
| Eu/GMP ICP | Has a self-adaptive property and rationally designing the competitive coordination interaction of Cu2þ between the guest CDs and Ab monomer. | From 40 to 50 | [22] | |
| Se−Cur/PLGA | Cur-loaded Se-PLGA nanosphere drug delivery system will decrease the amyloid-β load in the brain samples of AD mice and healed the model mice’s memory deficit substantially. | 160 ± 5 | [37] | |
| CdS | The biologically synthesized PC-metal nanoparticles, in particular iron oxide, do not impact neuroblastoma cells’ viability. | 50–60 | [24] | |
| DBP-PLGA | Significantly inhibited Aβ aggregation in vitro. Moreover, intravenous injection of DBP-PLGA nanoparticles significantly attenuated the Aβ accumulation, neuroinflammation, neuronal loss and cognitive dysfunction in the 5XFAD mice. | 226.6 ± 44.4 nm | [36] | |
| MEM–PEG–PLGA | Non-cytotoxic brain cell lines. Memantine adopted a slower release profile of NPs into the free medicine solution, minimizing the in vivo drug control frequency. | 200 | [38] | |
| PLGA-PEG-B6 | Could tremendously improve the spatial learning and memory capability of APP/PS1 mice, compared with native Cur | Less 100 | [42] | |
| Anti-Aβ1-42-NPs | Full memory defect correction; substantial decrease of the Aβ-soluble peptide and its brain oligomer level and significant increase of plasma Aβ levels. | 182 | [20] | |
| MoS2 QDs and TPP-MoS2 QDs | Exhibit a complete bifunctional nanozyme activity that prevents spontaneous neuroinflammation. | 30 and 50 | [30] | |
| Lipid nanoparticles | S80−, PS−, and PA-functionalized SLNs | Could ameliorate the cognition impairment of rats more effectively than the conventional administration of nicotinamide. | 112 ± 1.6, 124 ± 0.8, and 137 ± 1.05 | [65] |
| CN-SLNs | CN can be achieved therapeutically at lower doses and its oral bioavailability enhanced by encapsulating CN in SLNs. | 240.0 ± 4.79 | [67] | |
| TFB-SLNs | The therapeutic level of TFB could be transferred directly to the brain via the olfactory pathway, following the intranasal administration of polymers and lipid nanoparticles. | 200 | [68] | |
| SLN and NLC | NLC permeate more the blood–brain barrier, while amyloid-beta studies demonstrated NLC-transferrin has the capacity to inhibit fibril formation. | Lower than 250 | [39] | |
| EPO-SLN | High potential for drug encapsulation and improved anti-colon cell efficacy | 219.9 ± 15.6 | [69] | |
| NR C NRb Cb | The positive charge of the coating formula ensured that particles were mucoadhesive and that they were prolonged in the nasal cavity. | 335.76 ± 34.81 358.44 ± 25.89 419.47 ± 24.36 469.71 ± 49.07 | [47] | |
| Span 60 and cholesterol | Used to solve the problem of the extensive rapid metabolism of rivastigmine. | 100.7 | [48] | |
| Solid lipid nanoparticles (SLN) | Their efficacy, user-friendliness, versatility and intellectual property opportunities through innovating drug delivery in particular for drug release shift systems | 222 ± 21 to 414 ± 11 | [71] | |
| APOE-DONSLN | ApoE, which binds BBB receptors, can be used to successfully target solid lipid nanoparticles | 147.5 ± 0.8 | [50] | |
| NLC | Low toxicity and toxicity against the cell line SH-SY5Y | Below 200 | [75] | |
| RHT-SLNs | Improve the delivery of RHT brain targeting by producing and optimizing RHT-SLNs | 15.6 | [72] | |
| Lipid polymer hybrid NPs | Efficient, fast penetration into healthy albino rats of the bio-inspired surface-modified NPs | 111.6 ± 11.4 | [51] | |
| SLN and PLGA NPs | No toxicity, changes in body weight or clinical symptoms of the disease were found | 200 | [52] | |
| RT-loaded SLN | Zeta potential value of−10 mV was found, polydispersion index was found in the 0.3–0.6 range. | 214 | [73] | |
| RT loaded PLGA-Soya lecithin-Tween-80 | Therapeutic prospect to treat AD and potential carrier for providing sustained brain delivery of RT | 171.74 | [34] | |
| Curcumin and meloxicam-loaded lipid-core nanocapsules (LNC) | No toxicity in relation to the parameters determined of all LNC evaluated in mice | 424 nm (curcumin) and 365 nm (meloxicam) | [61] | |
| POPC: POPG 3:1 | Characterization of simultaneous size and zeta potential in individual capillary nanoparticles and particle mixtures under physiological salinities. | 76 ± 3 | [58] | |
| Gold nanoparticles | AuNPs | High functionality and high active area are used to improve the catalytic activity of captured AuNPs electrocatalytic tags. | 20 | [84] |
| Chiral l− and d−glutathione (GSH) stabilized Au NPs | Can inhibit Aβ42 aggregation and cross BBB after intravenous administration without substantial toxicity. | 3.3 | [85] | |
| D−/L−Pe−Au | Major decrease in the cell index, indicating that cytotoxic effects on PC12 cells depend on concentration. | 7 | [93] | |
| GNPs | Therapeutic ability of GNPs with behavioral and oxidative stress parameters in GNP-treated mice | 20 | [98] | |
| GNPs | Clinical potential may suppress CNS inflammation and oxidative stress, alleviating secondary neurodegenerative processes and reserpine-induced neuronal cell death. | 20 | [99] | |
| Pro-AuNPs nbbAuNPs | HEWL fibrillation greatly reduced with proline and pro-AuNP coincubation, and two slightly different intermediate species were produced with these two systems as CD spectroscopy predicts. | 529 nm and 523 nm | [100] | |
| PEG-coated AuNPs | PEG-coated gold anthocyanins nanoparticles may be a new therapeutic agent for neurodegenerative diseases | 135 ± 5 | [102] | |
| Citrate-based AuNPs | AuNPs SPR band intensity is susceptible to Aβ40 amyloids. This helps SPR test detect and semi-quantify Aβ40 amyloids and describe the kinetics of Aβ amyloid formation. | 23 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M. Potential Role of Nanoparticles in Treating the Accumulation of Amyloid-Beta Peptide in Alzheimer’s Patients. Polymers 2021, 13, 1051. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071051
Abbas M. Potential Role of Nanoparticles in Treating the Accumulation of Amyloid-Beta Peptide in Alzheimer’s Patients. Polymers. 2021; 13(7):1051. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071051
Chicago/Turabian StyleAbbas, Mohamed. 2021. "Potential Role of Nanoparticles in Treating the Accumulation of Amyloid-Beta Peptide in Alzheimer’s Patients" Polymers 13, no. 7: 1051. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071051