The Polymer-Based Technology in the Endovascular Treatment of Abdominal Aortic Aneurysms
Abstract
:1. Introduction
Abdominal Aortic Aneurysm Definition and Treatment Options
2. Polymer and Endovascular Aneurysm Repair
2.1. Concept of Traditional Endovascular Aneurysm Repair
2.2. Polyethylene Glycol: A New Tool for EVAR
- Biocompatibility: comprehensive biocompatibility testing was performed in accordance with the EN ISO 10993-1:2009/AC:2010 standard to evaluate the safety of the materials, including toxicity, sensitization, hemocompatibility, and irritation/intracutaneous reactivity (13-week intramuscular implant). All test results confirmed products are biocompatible and safe for implanting in humans.
- Bench testing: extensive engineering testing was performed on the bench to evaluate the design characteristics in accordance with the EN ISO 25539-1:2009 cardiovascular implants—endovascular devices—part 1: endovascular prostheses. All test results confirmed the products met their design specifications.
- Animal testing: chronic animal studies showed no evidence of structural changes in the fill polymer. A chronic animal study demonstrated no adverse effects associated with deliberate release of the fill polymer within the animals’ vasculature. In particular, no evidence of embolization, toxic response, or end-organ abnormality at 30 days was recorded.
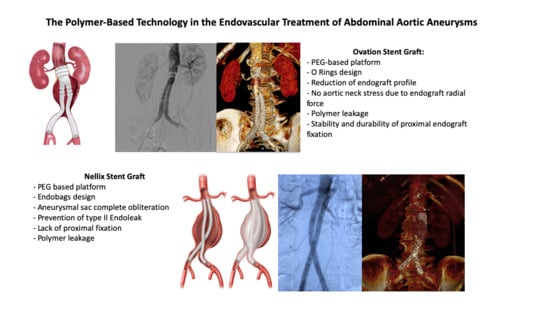
2.3. The Polymer-Filled Endobags, Endovascular Aneurysm Sealing (EVAS) with Nellix Stent Graft
2.4. The O-Ring EVAR Polymer-Based Proximal Neck Sealing Device, So-Called Ovation Stent Graft
3. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93, Erratum in 2020, 59, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.G.; Bown, M.J.; Glover, M.J.; Jones, E.; Masconi, K.L.; Michaels, J.A.; Powell, J.T.; Ulug, P.; Sweeting, M.J. Screening women aged 65 years or over for abdominal aortic aneurysm: A modelling study and health economic evaluation. Health Technol. Assess. 2018, 22, 1–142. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Williams, C.; Sweeting, M.J.; Turton, G.; Parkin, D.; Cooper, D.; Rodd, C.; Thompson, S.G.; Earnshaw, J.J.; Gloucestershire and Swindon Abdominal Aortic Aneurysm Screening Programme. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br. J. Surg. 2018, 105, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Schmitz-Rixen, T.; Keese, M.; Hakimi, M.; Peters, A.; Böckler, D.; Nelson, K.; Grundmann, R.T. Ruptured abdominal aortic aneurysm-epidemiology, predisposing factors, and biology. Langenbecks Arch. Surg. 2016, 401, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, L.B.; Maksic, M.; Koncar, I.; Ilic, N.; Dragas, M.; Fatic, N.; Markovic, M.; Banzic, I.; Mutavdzic, P. Open Repair of AAA in a High Volume Center. World J. Surg. 2017, 41, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Brooke, B.S. Effectiveness of open versus endovascular abdominal aortic aneurysm repair in population settings: A systematic review of statewide databases. Surgery 2017, 162, 707–720. [Google Scholar] [CrossRef]
- Suckow, B.D.; Goodney, P.P.; Columbo, J.A.; Kang, R.; Stone, D.H.; Sedrakyan, A.; Cronenwett, J.L.; Fillinger, M.F. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J. Vasc. Surg. 2018, 67, 1690–1697.e1. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, J.M.; Wood, E.A.; Slovut, D.P. Management of Aortic Aneurysms: Is Surgery of Historic Interest Only? Curr. Cardiol. Rep. 2015, 17, 105. [Google Scholar] [CrossRef]
- Setacci, C.; de Donato, G.; Setacci, F. Endografts for the treatment of aortic infection. Semin. Vasc. Surg. 2011, 24, 242–249. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Antoniou, S.A.; Torella, F. Editor’s Choice—Endovascular vs. Open Repair for Abdominal Aortic Aneurysm: Systematic Review and Meta-analysis of Updated Peri-operative and Long Term Data of Randomised Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhang, S.; Zhang, J.; Ji, C.; Eckstein, H.H. Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in Octogenarians: Meta-analysis and Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 454–463. [Google Scholar] [CrossRef] [Green Version]
- de Donato, G.; Setacci, C.; Chisci, E.; Setacci, F.; Giubbolini, M.; Sirignano, P.; Galzerano, G.; Cappelli, A.; Pieraccini, M.; Palasciano, G. Abdominal aortic aneurysm repair in octogenarians: Myth or reality? J. Cardiovasc. Surg. 2007, 48, 697–703. [Google Scholar]
- Pasqui, E.; de Donato, G.; Giannace, G.; Panzano, C.; Setacci, C.; Palasciano, G. Management of abdominal aortic aneurysm in nonagenarians: A single-centre experience. Vascular 2020, 1. [Google Scholar] [CrossRef]
- Sonesson, B.; Dias, N.; Resch, T. Is there an age limit for abdominal aortic aneurysm repair? J. Cardiovasc. Surg. 2018, 59, 190–194. [Google Scholar]
- Tan, T.W.; Eslami, M.; Rybin, D.; Doros, G.; Zhang, W.W.; Farber, A. Outcomes of endovascular and open surgical repair of ruptured abdominal aortic aneurysms in elderly patients. J. Vasc. Surg. 2017, 66, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Parodi, J.C. The challenges of medical innovation. J. Vasc. Surg. 2020, 71, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Veith, F.J.; Marin, M.L.; Cynamon, J.; Schonholz, C.; Parodi, J. 1992: Parodi, Montefiore, and the first abdominal aortic aneurysm stent graft in the United States. Ann. Vasc. Surg. 2005, 19, 749–751. [Google Scholar] [CrossRef]
- Ivancev, K.; Vogelzang, R. A 35 Year History of Stent Grafting, and How EVAR Conquered the World. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 685–694. [Google Scholar] [CrossRef]
- Kontopodis, N.; Papadopoulos, G.; Galanakis, N.; Tsetis, D.; Ioannou, C.V. Improvement of patient eligibility with the use of new generation endografts for the treatment of Abdominal Aortic Aneurysms. A comparison study among currently used endografts and literature review. Expert. Rev. Med. Devices 2017, 14, 245–250. [Google Scholar] [CrossRef]
- Moise, M.A.; Woo, E.Y.; Velazquez, O.C.; Fairman, R.M.; Golden, M.A.; Mitchell, M.E.; Carpenter, J.P. Barriers to endovascular aortic aneurysm repair: Past experience and implications for future device development. Vasc. Endovasc. Surg. 2006, 40, 197–203. [Google Scholar] [CrossRef]
- Krajcer, Z. TriVascular Ovation®: It’s role in solving current endograft deficiencies. J. Cardiovasc. Surg. 2015, 56, 325–329. [Google Scholar]
- Holden, A. Endovascular sac sealing concept: Will the Endologix Nellix™ device solve the deficiencies? J. Cardiovasc. Surg. 2015, 56, 339–353. [Google Scholar]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Becquemin, J.P.; Pillet, J.C.; Lescalie, F.; Sapoval, M.; Goueffic, Y.; Lermusiaux, P.; Steinmetz, E.; Marzelle, J. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J. Vasc. Surg. 2011, 53, 1167–1173.e1. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Kyriakides, T.C.; Stroupe, K.T.; Freischlag, J.A.; Padberg, F.T.J.; Matsumura, J.S.; Huo, Z.; Johnson, G.R. OVER Veterans Affairs Cooperative Study Group. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N. Engl. J. Med. 2019, 380, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Zarins, C.K.; AneuRx Clinical Investigators. The US AneuRx Clinical Trial: 6-year clinical update 2002. J. Vasc. Surg. 2003, 37, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Teijink, J.A.W.; Power, A.H.; Böckler, D.; Peeters, P.; van Sterkenburg, S.; Bouwman, L.H.; Verhagen, H.J.; Bosiers, M.; Riambau, V.; Becquemin, J.P.; et al. Editor’s Choice—Five Year Outcomes of the Endurant Stent Graft for Endovascular Abdominal Aortic Aneurysm Repair in the ENGAGE Registry. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Pinto, J.; Oliveira, N.F.G.; Bastos-Gonçalves, F.M.; Hoeks, S.; Rijn, M.J.V.; Raa, S.T.; Mansilha, A.; Verhagen, H.J.M. Long-term results after standard endovascular aneurysm repair with the Endurant and Excluder stent grafts. J. Vasc. Surg. 2020, 71, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Setacci, F.; Sirignano, P.; de Donato, G.; Chisci, E.; Iacoponi, F.; Galzerano, G.; Palasciano, G.; Cappelli, A.; Setacci, C. AAA with a challenging neck: Early outcomes using the Endurant stent-graft system. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Zandvoort, H.J.; Gonçalves, F.B.; Verhagen, H.J.; Werson, D.A.; Moll, F.L.; de Vries, J.P.; van Herwaarden, J.A. Results of endovascular repair of infrarenal aortic aneurysms using the Endurant stent graft. J. Vasc. Surg. 2014, 59, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Deery, S.E.; Shean, K.E.; Pothof, A.B.; O’Donnell, T.F.X.; Dalebout, B.A.; Darling, J.D.; Bodewes, T.C.F.; Schermerhorn, M.L. Three-Year Results of the Endurant Stent Graft System Post Approval Study. Ann. Vasc. Surg. 2018, 50, 202–208. [Google Scholar] [CrossRef]
- de Donato, G.; Setacci, F.; Bresadola, L.; Castelli, P.; Chiesa, R.; Mangialardi, N.; Nano, G.; Setacci, C. TriVascular Ovation Italian Study. Aortic neck evolution after endovascular repair with TriVascular Ovation stent graft. J. Vasc. Surg. 2016, 63, 8–15. [Google Scholar]
- Cao, P.; Verzini, F.; Parlani, G.; Rango, P.D.; Parente, B.; Giordano, G.; Giordano, G.; Mosca, S.; Maselli, A. Predictive factors and clinical consequences of proximal aortic neck dilatation in 230 patients undergoing abdominal aorta aneurysm repair with self-expandable stent-grafts. J. Vasc. Surg. 2003, 37, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Dillavou, E.D.; Muluk, S.; Makaroun, M.S. Is neck dilatation after endovascular aneurysm repair graft dependent? Results of 4 US Phase II trials. Vasc. Endovasc. Surg. 2005, 39, 47–54. [Google Scholar] [CrossRef]
- Cannavale, A.; Lucatelli, P.; Corona, M.; Nardis, P.; Cannavale, G.; De Rubeis, G.; Santoni, M.; Maher, B.; Catalano, C.; Bezzi, M. Current assessment and management of endoleaks after advanced EVAR: New devices, new endoleaks? Expert. Rev. Cardiovasc. Ther. 2020, 18, 465–473. [Google Scholar] [CrossRef]
- Chisci, E.; Setacci, F.; Iacoponi, F.; de Donato, G.; Cappelli, A.; Setacci, C. Surveillance imaging modality does not affect detection rate of asymptomatic secondary interventions following EVAR. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Spanos, K.; Rohlffs, F.; Panuccio, G.; Eleshra, A.; Tsilimparis, N.; Kölbel, T. Outcomes of endovascular treatment of endoleak type Ia after EVAR: A systematic review of the literature. J. Cardiovasc. Surg. 2019, 60, 175–185. [Google Scholar] [CrossRef]
- Chisci, E.; Kristmundsson, T.; de Donato, G.; Resch, T.; Setacci, F.; Sonesson, B.; Setacci, C.; Malina, M. The AAA with a challenging neck: Outcome of open versus endovascular repair with standard and fenestrated stent-grafts. J. Endovasc. Ther. 2009, 16, 137–146. [Google Scholar] [CrossRef]
- White, S.B.; Stavropoulos, S.W. Management of Endoleaks following Endovascular Aneurysm Repair. Semin. Intervent. Radiol. 2009, 26, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Quinn, A.A.; Mehta, M.; Teymouri, M.J.; Keenan, M.E.; Paty, P.S.K.; Zhou, Y.; Chang, B.B.; Feustel, P. The incidence and fate of endoleaks vary between ruptured and elective endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2017, 65, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, E.; Rubin, B.G.; Sanchez, L.A.; Choi, E.T.; Geraghty, P.J.; Baty, J.; Thompson, R.W.; Flye, M.W.; Hovsepian, D.M.; Picus, D.; et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: A conservative approach with selective intervention is safe and cost-effective. J. Vasc. Surg. 2004, 39, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Urquijo, M.; Lozano-Balderas, G.; Fabiani, M.A. Type II Endoleaks after EVAR: A Literature Review of Current Concepts. Vasc. Endovasc. Surg. 2020, 54, 718–724. [Google Scholar] [CrossRef]
- Sirignano, P.; Mansour, W.; Capoccia, L.; Cuozzo, S.; Camparini, S.; de Donato, G.; Mangialardi, N.; Ronchey, S.; Talarico, F.; Setacci, C.; et al. Immediate results of the expanding indications for treatment with standard EVAR in patients with challenging anatomies, a multi-centric prospective evaluation—EXTREME Study. Euro Interv. 2019. [Google Scholar] [CrossRef]
- Sirignano, P.; Mansour, W.; Capoccia, L.; Speziale, F. Rationale for a new registry on EVAR: The EXTREME study. Ann. Med. Surg. 2017, 21, 7–8. [Google Scholar] [CrossRef]
- Makaroun, M.S.; Deaton, D.H. Is proximal aortic neck dilatation after endovascular aneurysm exclusion a cause for concern? J. Vasc. Surg. 2001, 33, S39–S45. [Google Scholar] [CrossRef] [Green Version]
- Diehm, N.; Dick, F.; Katzen, B.T.; Schmidli, J.; Kalka, C.; Baumgartner, I. Aortic neck dilatation after endovascular abdominal aortic aneurysm repair: A word of caution. J. Vasc. Surg. 2008, 47, 886–892. [Google Scholar] [CrossRef] [Green Version]
- May, J.; White, G.H.; Ly, C.N.; Jones, M.A.; Harris, J.P. Endoluminal repair of abdominal aortic aneurysm prevents enlargement of the proximal neck: A 9-year life-table and 5-year longitudinal study. J. Vasc. Surg. 2003, 37, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Malas, M.B.; Ohki, T.; Veith, F.J.; Chen, T.; Lipsitz, E.C.; Shah, A.R.; Timaran, C.; Suggs, W.; Gargiulo, N.J., 3rd; Parodi, J.C. Absence of proximal neck dilatation and graft migration after endovascular aneurysm repair with balloon-expandable stent based endografts. J. Vasc. Surg. 2005, 42, 639–644. [Google Scholar] [CrossRef] [Green Version]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert. Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Putti, M.; Mes, T.; Huang, J.; Bosman, A.W.; Dankers, P.Y.W. Multi-component supramolecular fibers with elastomeric properties and controlled drug release. Biomater. Sci. 2019, 8, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Z.; You, M.L.; Ding, Z.Q.; Ye, W.B. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tay, R.; Khan, M.; Rachel Ee, P.L.; Hedrick, J.L.; Yang, Y.Y. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter. 2010, 6, 67–81. [Google Scholar] [CrossRef]
- Hutanu, D.; Frishberg, M.D.; Guo, L.; Darie, C.C. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 132. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. TriVascular, Inc. Ovation Abdominal Stent Graft System. Summary of Safety and Effectiveness Data (SSED). Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf12/P120006b.pdf (accessed on 9 May 2015).
- De Bruin, J.L.; Brownrigg, J.R.; Patterson, B.O.; Karthikesalingam, A.; Holt, P.J.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. The Endovascular Sealing Device in Combination with Parallel Grafts for Treatment of Juxta/Suprarenal Abdominal Aortic Aneurysms: Short-term Results of a Novel Alternative. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Trellopoulos, G.; Georgakarakos, E.; Pelekas, D.; Papachristodoulou, A.; Kalaitzi, A.; Asteri, T. Initial single-center experience with the Ovation stent-graft system in the treatment of abdominal aortic aneurysms: Application to challenging iliac access anatomies. Ann. Vasc. Surg. 2015, 29, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Nano, G.; Mazzaccaro, D.; Stegher, S.; Occhiuto, M.T.; Malacrida, G.; Tealdi, D.G.; Alberti, A.; Volpe, P. Early experience with Ovation endograft system in abdominal aortic disease. J. Cardiothorac. Surg. 2014, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Choo, X.Y.; Hajibandeh, S.; Hajibandeh, S.; Antoniou, G.A. The Nellix endovascular aneurysm sealing system: Current perspectives. Med. Devices 2019, 12, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boersen, J.T. Validation of Endovascular Aneurysm Sealing for Treatment of Abdominal Aortic Aneurysm. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2017. [Google Scholar] [CrossRef] [Green Version]
- Karthikesalingam, A.; Cobb, R.J.; Khoury, A.; Choke, E.C.; Sayers, R.D.; Holt, P.J.; Thompson, M.M. The morphological applicability of a novel endovascular aneurysm sealing (EVAS) system (Nellix) in patients with abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Böckler, D.; Holden, A.; Thompson, M.; Hayes, P.; Krievins, D.; de Vries, J.P.; Reijnen, M.M. Multicenter Nellix EndoVascular Aneurysm Sealing system experience in aneurysm sac sealing. J. Vasc. Surg. 2015, 62, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Silingardi, R.; Coppi, G.; Ferrero, E.; Lauricella, A.; Psacharopulo, D.; Saitta, G.; Viazzo, A.; Ferri, M. Midterm Outcomes of the Nellix Endovascular Aneurysm Sealing System: A Dual-Center Experience. J. Endovasc. Ther. 2016, 23, 695–700. [Google Scholar] [CrossRef]
- Gossetti, B.; Martinelli, O.; Ferri, M.; Silingardi, R.; Verzini, F.; IRENE Group Investigators. Preliminary results of endovascular aneurysm sealing from the multicenter Italian Research on Nellix Endoprosthesis (IRENE) study. J. Vasc. Surg. 2018, 67, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglino, S.; Mortola, L.; Ferrero, E.; Ferri, M.; Cirillo, S.; Lario, C.V.; Negro, G.; Ricotti, A.; Gaggiano, A. Long-term failure after EVAS in a real-life single center experience with the Nellix endograft. J. Vasc. Surg. 2020, 2. [Google Scholar] [CrossRef]
- Carpenter, J.P.; Lane, J.S., 3rd; Trani, J.; Hussain, S.; Healey, C.; Buckley, C.J.; Hashemi, H.; Cuff, R.; Nellix Investigators. Refinement of anatomic indications for the Nellix System for endovascular aneurysm sealing based on 2-year outcomes from the EVAS FORWARD IDE trial. J. Vasc. Surg. 2018, 68, 720–730. [Google Scholar] [PubMed]
- Stenson, K.M.; de Bruin, J.L.; Loftus, I.M.; Holt, P.J.E. Migration and sac expansion as modes of midterm therapeutic failure after endovascular aneurysm sealing. J. Vasc. Surg. 2020, 71, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.A.; Benaragama, K.S.; Pope, T.; Coughlin, P.A.; Winterbottom, A.P.; Harrison, S.C.; Boyle, J.R. Progressive Device Failure at Long Term Follow Up of the Nellix EndoVascular Aneurysm Sealing (EVAS) System. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 211–218. [Google Scholar] [CrossRef]
- Zoethout, A.C.; Zerwes, S.; Zeebregts, C.J.A.M.; Heyligers, J.M.M.; De Vries, J.P.J.M.; Oberhuber, A.; Karl, T.; Berg, P.; Stenson, K.; Loftus, I.; et al. Preliminary outcome of Nellix-in-Nellix extensions in patients treated with failed endovascular aneurysm sealing. J. Vasc. Surg. 2019, 70, 1099–1106. [Google Scholar] [CrossRef]
- Harrison, S.C.; Winterbottom, A.J.; Coughlin, P.A.; Hayes, P.D.; Boyle, J.R. Editor’s Choice—Mid-term Migration and Device Failure Following Endovascular Aneurysm Sealing with the Nellix Stent Graft System—A Single Centre Experience. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Endologix Provides An Update on the Previously Announced Voluntary Nellix System Recall. Available online: https://endologix.com/wp-content/uploads/2019/01/2019.01.22-Press-ReleaseNellix-CE-Mark-FINAL.pdf (accessed on 15 January 2021).
- Hynecek, R.L.; Sadek, M.; Derubertis, B.G.; Ryer, E.J.; Choi, J.; Hsu, S.; Kent, K.C.; Faries, P.L. Evaluation of pressure transmission and intra-aneurysmal contents after endovascular repair using the Trivascular Enovus expanded polytetrafluoroethylene stent graft in a canine model of abdominal aortic aneurysm. J. Vasc. Surg. 2007, 46, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Faries, P.L.; Dayal, R.; Rhee, J.; Trocciola, S.; Kent, K.C. Stent graft treatment for abdominal aortic aneurysm repair: Recent developments in therapy. Curr. Opin. Cardiol. 2004, 19, 551–557. [Google Scholar] [CrossRef]
- Moulakakis, K.G.; Dalainas, I.; Kakisis, J.; Giannakopoulos, T.G.; Liapis, C.D. Current knowledge on EVAR with the ultra-low profile Ovation Abdominal Stent-graft System. J. Cardiovasc. Surg. 2012, 53, 427–432. [Google Scholar]
- Sirignano, P.; Capoccia, L.; Menna, D.; Mansour, W.; Speziale, F. Pushing forward the limits of EVAR: New therapeutic solutions for extremely challenging AAAs using the Ovation Stent Graft. J. Cardiovasc. Surg. 2016, 57, 839–845. [Google Scholar]
- de Donato, G.; Setacci, F.; Sirignano, P.; Galzerano, G.; Borrelli, M.P.; di Marzo, L.; Setacci, C. Ultra-low profile Ovation device: Is it the definitive solution for EVAR? J. Cardiovasc. Surg. 2014, 55, 33–40. [Google Scholar]
- Georgakarakos, E.; Ioannou, C.V.; Georgiadis, G.S.; Storck, M.; Trellopoulos, G.; Koutsias, S.; Miltos, K.L. The ovation abdominal stent graft for the treatment of abdominal aortic aneurysms: Current evidence and future perspectives. Expert Rev. Med. Devices 2016, 13, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Georgakarakos, E.; Papatheodorou, N.; Argyriou, C.; Tasopoulou, K.M.; Doukas, D.; Georgiadis, G.S. An update on the ovation abdominal stent graft for the treatment of abdominal aortic aneurysms: Current evidence and future perspectives. Expert Rev. Med. Device 2020, 17, 1249–1256. [Google Scholar] [CrossRef]
- Sweet, M.P.; Fillinger, M.F.; Morrison, T.M.; Abel, D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 931–937. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.; Valdés, F.E.; Nolte, T.; Mishkel, G.J.; Jordan, W.D.; Gray, B.; Eskandari, M.K.; Botti, C. A Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the Ovation Abdominal Stent Graft System Investigators. One-year outcomes from an international study of the Ovation Abdominal Stent Graft System for endovascular aneurysm repair. J. Vasc. Surg. 2014, 59, 65–73.e3. [Google Scholar] [CrossRef] [Green Version]
- Varkevisser, R.R.B.; Swerdlow, N.J.; Verhagen, H.J.M.; Lyden, S.P.; Schermerhorn, M.L. Similar 5-year outcomes between female and male patients undergoing elective endovascular abdominal aortic aneurysm repair with the Ovation stent graft. J. Vasc. Surg. 2020, 72, 114–121. [Google Scholar] [CrossRef]
- Savlovskis, J.; Krievins, D.; de Vries, J.P.; Holden, A.; Kisis, K.; Gedins, M.; Ezite, N.; Zarins, C.K. Aortic neck enlargement after endovascular aneurysm repair using balloon-expandable versus self-expanding endografts. J. Vasc. Surg. 2015, 62, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Ierardi, A.M.; Tsetis, D.; Ioannou, C.; Laganà, D.; Floridi, C.; Petrillo, M.; Pinto, A.; Piffaretti, G.; Carrafiello, G. Ultra-low profile polymer-filled stent graft for abdominal aortic aneurysm treatment: A two-year follow-up. Radiol. Med. 2015, 120, 542–548. [Google Scholar] [CrossRef]
- Carrafiello, G.; Ierardi, A.; Piffaretti, G.; Rivolta, N.; Floridi, C.; Aswad, A.; Della Valle, F.; Ioannou, C.V.; Gentilini, C.; Tsetis, D.; et al. Treatment of abdominal aortic aneurysm with a new type of polymer-filled low profile device. Int. J. Surg. 2013, 11, S24–S29. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, N.J.; Lyden, S.P.; Verhagen, H.J.M.; Schermerhorn, M.L. Five-year reslts of endovascular abdominal aortic aneurysm repair with the Ovation abdominal stent graft. J. Vasc. Surg. 2020, 71, 1528–1537.e2. [Google Scholar] [CrossRef]
- de Donato, G.; Setacci, F.; Bresadola, L.; Castelli, P.; Chiesa, R.; Mangialardi, N.; Nano, G.; Stetacci, C. Midterm Results of Proximal Aneurysm Sealing With the Ovation Stent-Graft According to On- vs. Off-Label Use. J. Endovasc. Ther. 2017, 24, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Speziale, F.; Sirignano, P.; Setacci, F.; Menna, D.; Capoccoa, L.; Mansour, W.; Galzerano, G.; Setacci, C. Immediate and two-year outcomes after EVAR in “on-label” and “off-label” neck anatomies using different commercially available devices. analysis of the experience of two Italian vascular centers. Ann. Vasc. Surg. 2014, 28, 1892–1900. [Google Scholar] [CrossRef]
- Crawford, E.S.; Beckett, W.C.; Greer, M.S. Juxtarenal infrarenal abdominal aortic aneurysm. Special diagnostic and therapeutic considerations. Ann. Surg. 1986, 203, 661–670. [Google Scholar] [CrossRef]
- de Donato, G.; Pasqui, E.; Mele, M.; Panzano, C.; Giannace, G.; Setacci, F.; Benevento, D.; Setacci, C.; Palasciano, G. The use of a low-profile stent graft with a polymer ring sealing technology combined with bare renal stent (vent technique) in patients with juxtarenal aneurysm not eligible for open surgery and fenestrated endograft. J. Vasc. Surg. 2020, 71, 1843–1850. [Google Scholar] [CrossRef]
- Volpe, P.; Alberti, A.; Massara, M. Endovascular Treatment of Juxtarenal Abdominal Aortic Aneurysms Recurring to the “Vent Technique”. Ann. Vasc. Surg. 2020, 68, 270–274. [Google Scholar] [CrossRef]
- Kret, M.R.; Tran, K.; Lee, J.T. Change in Aortic Neck Diameter after Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2017, 43, 115–120. [Google Scholar] [CrossRef]
- Tassiopoulos, A.K.; Monastiriotis, S.; Jordan, W.D.; Muhs, B.E.; Ouriel, K.; De Vries, J.P. Predictors of early aortic neck dilatation after endovascular aneurysm repair with EndoAnchors. J. Vasc. Surg. 2017, 66, 45–52. [Google Scholar] [CrossRef]
- Ribner, A.S.; Tassiopoulos, A.K. Postoperative Aortic Neck Dilation: Myth or Fact? Int. J. Angiol. 2018, 27, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Kouvelos, G.N.; Oikonomou, K.; Antoniou, G.A.; Verhoeven, E.L.; Katsargyris, A. A systematic review of proximal neck dilatation after endovascular repair for abdominal aortic aneurysm. J. Endovasc. Ther. 2017, 24, 59–67. [Google Scholar] [CrossRef]
- Holden, A.; Lyden, S. Initial experience with polymer endovascular aneurysm repair using the Alto stent graft. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Siani, A.; Accrocca, F.; De Vivo, G.; Mounayergi, F.; Siani, L.M.; Antonelli, R. Anaphylactic Reaction during Implantation of Ovation Abdominal Stent Graft in Patients with Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2017, 39, 289.e1–289.e4. [Google Scholar] [CrossRef]
- Sfyroeras, G.S.; Moulakakis, K.G.; Antonopoulos, C.N.; Manikis, D.; Vasdekis, S.N. Anaphylactic Reaction During Implantation of the Ovation Stent-Graft System in a Patient With Abdominal Aortic Aneurysm. J. Endovasc. Ther. 2015, 22, 620–622. [Google Scholar] [CrossRef]
- Simonte, G.; Isernia, G.; Fiorucci, B.; Paciaroni, E.; Cieri, E.; Rebonato, A.; Lenti, M. Polymer Embolization and Anaphylactic Reaction during Implantation of an Ovation Stent Graft for Abdominal Aortic Aneurysm Exclusion. Ann. Vasc. Surg. 2018, 50, e7–e298. [Google Scholar] [CrossRef]
- Uchida, K.; Yamato, M.; Ito, E.; Kwon, O.H.; Kikuchi, A.; Sakai, K.; Okano, T. Two different types of nonthrombogenic surfaces: PEG suppresses platelet adhesion ATP-independently but HEMA-St block copolymer requires ATP consumption of platelets to prevent adhesion. J. Biomed. Mater. Res. 2000, 50, 585–590. [Google Scholar] [CrossRef]
- Bjugstad, K.B.; Lampe, K.; Kern, D.S.; Mahoney, M. Biocompatibility of poly (ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J. Biomed. Mater. Res. Part. A 2010, 95, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.T.H.; Helder, M.; Russell, R.A.; Holden, P.J.; Foster, L.J.R. Application of Polyethylene Glycol to Promote Cellular Biocompatibility of Polyhydroxybutyrate Films. Int. J. Polym. Sci. 2011, 2011, 473045. [Google Scholar] [CrossRef]
- Macy, E.M. Current Epidemiology and Management of Radiocontrast-Associated Acute- and Delayed-Onset Hypersensitivity: A Review of the Literature. Perm. J. 2018, 22, 17-072. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Hynes, K.L.; Mahrouyan, O.; Briggs, C.S.; Azzizadeh, A. Polymer leak with the Ovation Abdominal Stent Graft System: Early recognition and treatment. Vascular 2020, 28, 159–164. [Google Scholar] [CrossRef] [PubMed]




| Type of Endograft | Type of Polymer Used | Type of Endograft Fixation | Polymer Location | Main Advantages | Eventual Critical Issues |
|---|---|---|---|---|---|
| Ovation stent graft | PEG | Proximal | O-rings network | -Reduction of endograft profile -No aortic neck stress due to endograft radial force | -Polymer leakage -Stability and durability of proximal endograft fixation |
| Nellix stent graft | PEG | Aneurysm sac obliteration | Endobags | -Aneurysmal sac complete obliteration -Prevention of type II EL | -Lack of proximal fixation -Polymer leakage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Donato, G.; Pasqui, E.; Panzano, C.; Brancaccio, B.; Grottola, G.; Galzerano, G.; Benevento, D.; Palasciano, G. The Polymer-Based Technology in the Endovascular Treatment of Abdominal Aortic Aneurysms. Polymers 2021, 13, 1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081196
de Donato G, Pasqui E, Panzano C, Brancaccio B, Grottola G, Galzerano G, Benevento D, Palasciano G. The Polymer-Based Technology in the Endovascular Treatment of Abdominal Aortic Aneurysms. Polymers. 2021; 13(8):1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081196
Chicago/Turabian Stylede Donato, Gianmarco, Edoardo Pasqui, Claudia Panzano, Brenda Brancaccio, Gaia Grottola, Giuseppe Galzerano, Domenico Benevento, and Giancarlo Palasciano. 2021. "The Polymer-Based Technology in the Endovascular Treatment of Abdominal Aortic Aneurysms" Polymers 13, no. 8: 1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081196