Designing Biodegradable and Active Multilayer System by Assembling an Electrospun Polycaprolactone Mat Containing Quercetin and Nanocellulose between Polylactic Acid Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
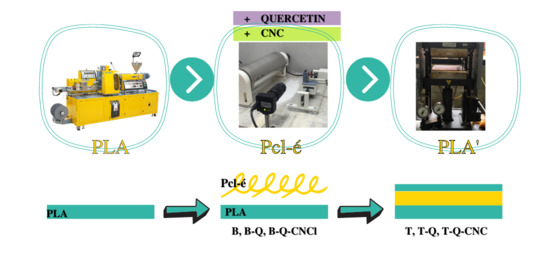
2.2. Preparation of Quercetin-Containing Multilayer Systems
2.3. Characterization of Physical Properties
2.3.1. Optical Parameter of Trilayer Systems
2.3.2. Microstructural Analysis of Trilayer Systems
2.3.3. Thermal Properties of Bi- and Trilayer Systems
2.3.4. Water Vapor Permeability (WVP) Analysis
2.4. Specific Migration Studies
2.5. Statistical Analysis
3. Results
3.1. Optical Properties
3.2. Morphological Analysis of Developed Trilayer Films
3.3. Thermal Properties
3.4. Water Vapor Barrier Properties
3.5. Quercetin Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Rydz, J.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Sikorska, W. Present and Future of Biodegradable Polymers for Food Packaging Applications. Biopolym. Food Des. 2018, 431–467. [Google Scholar] [CrossRef]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Sheng, K.; Yu, K.; Xu, L.; Fontanillo Lopez, C.A. Improved properties of PLA biocomposites toughened with bamboo cellulose nanowhiskers through silane modification. J. Mater. Sci. 2018, 53, 10920–10932. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López de Dicastillo, C.; Roa, K.; Garrido, L.; Pereira, A.; Galotto, M. Novel Polyvinyl Alcohol/Starch Electrospun Fibers as a Strategy to Disperse Cellulose Nanocrystals into Poly(lactic acid). Polymers 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Torres-Giner, S.; Lagaron, J.M. Chapter 6—Nanostructured Multilayer Films. In Micro and Nano Technologies; Cerqueira, M.Â.P.R., Lagaron, J.M., Pastrana Castro, L.M., de Oliveira Soares Vicente, A.A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–171. ISBN 978-0-323-51271-8. [Google Scholar]
- Messin, T.; Marais, S.; Follain, N.; Guinault, A.; Gaucher, V.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS multinanolayer membrane with enhanced barrier performances. J. Memb. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Gu, C.H.; Wang, J.J.; Yu, Y.; Sun, H.; Shuai, N.; Wei, B. Biodegradable multilayer barrier films based on alginate/polyethyleneimine and biaxially oriented poly(lactic acid). Carbohydr. Polym. 2013, 92, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, Y.; Bhattacharyya, D.; Sui, G. Novel sandwiched structures in starch/cellulose nanowhiskers (CNWs) composite films. Compos. Commun. 2017, 4, 5–9. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Zhao, R.; Zhou, Y.; Wang, Z. Preparation of nanofibrillated cellulose and application in reinforced PLA/starch nanocomposite film. J. Polym. Environ. 2019, 27, 728–738. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H. Novel techniques in food processing: Bionanocomposites. Curr. Opin. Food Sci. 2018, 23, 49–56. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Bari, S.S.; Chatterjee, A.; Mishra, S. Biodegradable polymer nanocomposites: An overview. Polym. Rev. 2016, 56, 287–328. [Google Scholar] [CrossRef]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 106747. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Jacob, J.; Thomas, S.; Loganathan, S.; Valapa, R.B. Antioxidant incorporated biopolymer composites for active packaging. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–260. [Google Scholar]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Wang, L.; Yang, X.; Guo, J.; Yin, S. Enhanced water resistance properties of bacterial cellulose multilayer films by incorporating interlayers of electrospun zein fibers. Food Hydrocoll. 2016, 61, 269–276. [Google Scholar] [CrossRef]
- Arrieta, M.P.; García, A.D.; López, D.; Fiori, S.; Peponi, L. Antioxidant bilayers based on PHBV and plasticized electrospun PLA-PHB fibers encapsulating catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yan, Y.; Guan, X.; Huang, K. Preparation of a hordein-quercetin-chitosan antioxidant electrospun nanofibre film for food packaging and improvement of the film hydrophobic properties by heat treatment. Food Packag. Shelf Life 2020, 23, 100466. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al 3+ -sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M.d.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, E.; Rojas, A.; Piña, C.; Galotto, M.J.; López de Dicastillo, C. Development of bilayer biodegradable composites containing cellulose nanocrystals with antioxidant properties. Polymers 2019, 11, 1945. [Google Scholar] [CrossRef] [Green Version]
- López de Dicastillo, C.; Alonso, J.; Catalá, R.; Gavara, R.; Hernández-Munoz, P. Improving the antioxidant protection of packaged food by incorporating natural flavonoids into ethylene-vinyl alcohol copolymer (EVOH) films. J. Agric. Food Chem. 2010, 58. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. Development of Chitosan and Polycaprolactone based active bilayer films enhanced with nanocellulose and grape seed extract. Carbohydr. Polym. 2018, 195, 180–188. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Enhancing the functional properties of acetylated hemicellulose films for active food packaging using acetylated nanocellulose reinforcement and polycaprolactone coating. Food Packag. Shelf Life 2020, 24, 100481. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. Biodegradation of Biopolymers. In Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products; Elsevier: Amsterdam, The Netherlands, 2016; pp. 739–755. ISBN 9780444636621. [Google Scholar]
- Velásquez, E.; Garrido, L.; Valenzuela, X.; Galotto, M.J.; Guarda, A.; López de Dicastillo, C. Physical properties and safety of 100% post-consumer PET bottle -organoclay nanocomposites towards a circular economy. Sustain. Chem. Pharm. 2020, 17, 100285. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Garrido, L.; Galotto, M.J.; López de Dicastillo, C. Design of active electrospun mats with single and core-shell structures to achieve different curcumin release kinetics. J. Food Eng. 2020, 273. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of molecular weight on the crystallization kinetics of polycaprolactone. Polym. Adv. Technol. 2006, 17, 474–478. [Google Scholar] [CrossRef]
- ISO—ISO 2528:1995—Sheet Materials—Determination of Water Vapour Transmission Rate—Gravimetric (Dish) Method. Available online: https://www.iso.org/standard/20676.html (accessed on 18 March 2021).
- Feigenbaum, A.E.; Riquet, A.M.; Scholler, D. Fatty food simulants: Solvents to mimic the behavior of fats in contact with packaging plastics. ACS Symp. Ser. 2000, 753, 71–81. [Google Scholar] [CrossRef]
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the Food Contact Suitability of Aged Bio-Nanocomposite Materials Dedicated to Food Packaging Applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef] [Green Version]
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R.; Rollini, M. Development of a novel antimicrobial film based on chitosan with LAE (ethyl-Nα-dodecanoyl-l-arginate) and its application to fresh chicken. Int. J. Food Microbiol. 2013, 165, 339–345. [Google Scholar] [CrossRef]
- Rojas, A.; Velásquez, E.; Piña, C.; Galotto, M.J.; López de Dicastillo, C. Designing active mats based on cellulose acetate/polycaprolactone core/shell structures with different release kinetics. Carbohydr. Polym. 2021, 261, 117849. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Massek, A. Impregnation of Poly(lactic Acid) with Polyphenols of Plant Origin. Fibres Text. East. Eur. 2020, 28, 15–20. [Google Scholar] [CrossRef]
- Bi, H.; Ren, Z.; Ye, G.; Sun, H.; Guo, R.; Jia, X.; Xu, M. Fabrication of cellulose nanocrystal reinforced thermoplastic polyurethane/polycaprolactone blends for three-dimension printing self-healing nanocomposites. Cellulose 2020, 27, 8011–8026. [Google Scholar] [CrossRef]
- Bassi, A.K.; Gough, J.E.; Zakikhani, M.; Downes, S. The Chemical and Physical Properties of Poly(ε-caprolactone) Scaffolds Functionalised with Poly(vinyl phosphonic acid-co-acrylic acid). J. Tissue Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Herrera-Kao, W.A.; Loría-Bastarrachea, M.I.; Pérez-Padilla, Y.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Cervantes-Uc, J.M. Thermal degradation of poly(caprolactone), poly(lactic acid), and poly(hydroxybutyrate) studied by TGA/FTIR and other analytical techniques. Polym. Bull. 2018, 75, 4191–4205. [Google Scholar] [CrossRef]
- Milia, A.; Bruno, M.; Cavallaro, G.; Lazzara, G.; Milioto, S. Adsorption isotherms and thermal behavior of hybrids based on quercetin and inorganic fillers. J. Therm. Anal. Calorim. 2019, 1–7. [Google Scholar] [CrossRef]
- Muthurajan, T.; Rammanohar, P.; Rajendran, N.P.; Sethuraman, S.; Krishnan, U.M. Evaluation of a quercetin–gadolinium complex as an efficient positive contrast enhancer for magnetic resonance imaging. RSC Adv. 2015, 5, 86967–86979. [Google Scholar] [CrossRef]
- Vaz, G.R.; Clementino, A.; Bidone, J.; Villetti, M.A.; Falkembach, M.; Batista, M.; Barros, P.; Sonvico, F.; Dora, C. Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials 2020, 10, 1650. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131. [Google Scholar] [CrossRef]




| Films | Thickness (µm) | L* | a* | b* | ΔE* | Opacity |
|---|---|---|---|---|---|---|
| PLA | 79.4 ± 6.9 a | 98.2 ± 0.1 d | −0.05 ± 0.01 f | 2.2 ± 0.1 a | – | 0.6 ± 0.1 a |
| B | 81.3 ± 6.8 a | 97.5 ± 0.1 b | −0.09 ± 0.01 e | 2.4 ± 0.1 c | 0.8 ± 0.1 b | 12.8 ± 1.3 d |
| B-Q | 83.6 ± 8.9 a | 97.1 ± 0.1 a | −0.51 ± 0.03 c | 3.4 ± 0.1 e | 1.7 ± 0.1 d | 7.4 ± 2.1 c |
| B-Q-CNC | 83.5 ± 8.1 a | 97.2 ± 0.1 a | −0.44 ± 0.02 d | 3.2 ± 0.1 d | 1.5 ± 0.1 c | 7.2 ± 1.9 c |
| T | 99.0 ± 3.5 a | 98.3 ± 0.1 d | −0.11 ± 0.01 e | 2.3 ± 0.1 b | 0.2 ± 0.1 a | 3.7 ± 0.3 b |
| T-Q | 102.2 ± 5.2 b | 97.9 ± 0.1 c | −0.57 ± 0.02 b | 3.9 ± 0.1 f | 1.9 ± 0.1 e | 3.6 ± 0.6 b |
| T-Q-CNC | 103.7 ± 4.3 b | 97.9 ± 0.1 c | −0.61 ± 0.02 a | 3.9 ± 0.1 f | 1.9 ± 0.1 e | 3.1 ± 0.2 b |
| Films | Tg (°C) | Tcc (°C) | ΔHcc (J g−1) | Tm1 (°C) | Tm2 (°C) | ΔHm (J g−1) | Xc (%) |
|---|---|---|---|---|---|---|---|
| PCLé | – | – | – | 59.2 ± 0.8 a | – | 108.7 ± 1.1 b | 78.0 ± 0.8 e |
| PLA film | 63.5 ± 0.1 b | 114.7 ± 0.1 bc | 37.3 ± 0.5 b | 149.2 ± 0.1 c | 153.9 ± 0.3 b | 39.2 ± 0.1 a | 2.0 ± 0.6 ab |
| B | 63.2 ± 0.5 b | 115.1 ± 0.4 c | 36.6 ± 0.3 b | 149.1 ± 0.4 c | 153.6 ± 0.2 b | 38.4 ± 0.3 a | 1.9 ± 0.1 ab |
| B-Q | 63.1 ± 0.3 b | 115.2 ± 0.2 c | 33.2 ± 4.1 b | 149.3 ± 0.1 c | 153.8 ± 0.1 b | 36.5 ± 3.9 a | 3.6 ± 0.2 b |
| B-Q-CNC | 63.1 ± 0.2 b | 114.9 ± 0.1 bc | 36.5 ± 1.5 b | 148.9 ± 0.2 c | 153.6 ± 0.2 b | 37.4 ± 1.7 a | 1.0 ± 0.2 a |
| T | 61.1 ± 0.1 a | 114.5 ± 0.2 ab | 20.1 ± 2.1 a | 147.4 ± 0.3 b | 152.3 ± 0.2 a | 38.1 ± 0.6 a | 19.1 ± 1.6 d |
| T-Q | 61.1 ± 0.1 a | 113.9 ± 0.1 a | 23.2 ± 0.2 a | 147.1 ± 0.2 b | 152.0 ± 0.3 a | 38.8 ± 0.1 a | 16.7 ± 0.1 c |
| T-Q-CNC | 60.5 ± 0.7 a | 114.0 ± 0.5 a | 23.7 ± 2.1 a | 147.6 ± 0.9 b | 151.6 ± 0.7 a | 37.4 ± 0.4 a | 14.7 ± 1.8 c |
| Films | Tonset (°C) | Td,max (°C) | Mass Loss at Td,max (wt%) |
|---|---|---|---|
| PLA film | 335.1 | 364.3 | 56.4 |
| PCLé fiber | 351.4 | 412.7 | 56.5 |
| B | 335.7 | 364.3 | 56.3 |
| B-Q | 330.9 | 363.2 | 56.5 |
| B-Q-CNC | 330.4 | 366.1 | 59.2 |
| T | 309.5 | 363.6 | 59.3 |
| T-Q | 304.8 | 363.7 | 61.1 |
| T-Q-CNC | 305.5 | 364.3 | 61.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Dicastillo, C.; Garrido, L.; Velásquez, E.; Rojas, A.; Gavara, R. Designing Biodegradable and Active Multilayer System by Assembling an Electrospun Polycaprolactone Mat Containing Quercetin and Nanocellulose between Polylactic Acid Films. Polymers 2021, 13, 1288. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081288
López de Dicastillo C, Garrido L, Velásquez E, Rojas A, Gavara R. Designing Biodegradable and Active Multilayer System by Assembling an Electrospun Polycaprolactone Mat Containing Quercetin and Nanocellulose between Polylactic Acid Films. Polymers. 2021; 13(8):1288. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081288
Chicago/Turabian StyleLópez de Dicastillo, Carol, Luan Garrido, Eliezer Velásquez, Adrián Rojas, and Rafael Gavara. 2021. "Designing Biodegradable and Active Multilayer System by Assembling an Electrospun Polycaprolactone Mat Containing Quercetin and Nanocellulose between Polylactic Acid Films" Polymers 13, no. 8: 1288. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13081288