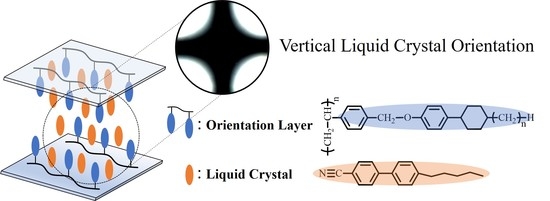
Vertical Orientation of Liquid Crystal on Comb-Like 4-(trans-4-alkylcyclohexyl)phenoxymethyl-substituted Polystyrene Containing Liquid Crystal Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 4-(trans-4-alkylcyclohexyl)phenoxymethyl Modified Polystyrene
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scharf, T. Polarized Light in Liquid Crystals and Polymers, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 103–141. [Google Scholar]
- Hamley, I.W. Introduction to Soft Matter: Polymers, Colloids, Amphiphiles, and Liquid Crystals, 1st ed.; John Wiley & Sons: West Sussex, UK, 2000; pp. 267–311. [Google Scholar]
- Khoo, C.H.; Simoni, F. Physics of Liquid Crystalline Materials, 1st ed.; Gordon & Breach Publishers: Philadelphia, PA, USA, 1991; pp. 3–29. [Google Scholar]
- De Gennes, P.G. The Physics of Liquid Crystals, 1st ed.; Oxford University Press: Oxford, UK, 1974; pp. 1–18, 23–50. [Google Scholar]
- Chandrasekhar, S. Liquid Crystals, 1st ed.; Cambridge University Press: Cambridge, UK, 2010; pp. 1–84. [Google Scholar]
- Kim, D.; Jahn, A.; Cho, S.; Kim, J.S.; Ki, M.; Kim, D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar]
- Mezzenga, R.; Schurtenberger, P.; Burbidge, A.; Michel, M. Understanding foods as soft materials. Nat. Mater. 2005, 4, 729–740. [Google Scholar]
- Drummond, C.J.; Fong, C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr. Opin. Colloid Interface Sci. 1999, 4, 449–456. [Google Scholar]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar]
- Clogston, J.; Caffrey, M. Controlling release from the lipidic cubic phase. amino acids, peptides, proteins and nucleic acids. J. Control. Release 2005, 107, 97–111. [Google Scholar]
- Ubbink, J.; Burbidge, A.; Mezzenga, R. Food structure and functionality: A soft matter perspective. Soft Matter 2008, 4, 1569–1581. [Google Scholar]
- Mohammady, S.Z.; Pouzot, M.; Mezzenga, R. Oleoylethanolamide-based lyotropic liquid crystals as vehicles for delivery of amino acids in aqueous environment. Biophys. J. 2009, 96, 1537–1546. [Google Scholar]
- Komisarski, M.; Osornio, Y.M.; Siegel, J.S.; Landau, E.M. Tailored host–guest lipidic cubic phases: A protocell model exhibiting nucleic acid recognition. Chem. Eur. J. 2013, 19, 1262–1267. [Google Scholar]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mat. Chem. B 2017, 5, 5061–5078. [Google Scholar]
- Collings, P.J.; Goodby, J.W. Introduction to Liquid Crystals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 29–84. [Google Scholar]
- Ye, L.; Zhao, C.; Feng, Y.; Gu, B.; Cui, Y.; Lu, Y. Study on the polarization of random lasers from dye-doped nematic liquid crystals. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar]
- Stöhr, J.; Samant, M.G.; Cossy-Favre, A.; Diaz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar]
- Xia, C.; Zhou, D.; Su, Y.; Zhou, G.; Yao, L.; Sun, W.; Liu, Y. A liquid-crystal-based immunosensor for the detection of cardiac troponin I. Analyst 2020, 145, 4569–4575. [Google Scholar]
- Sivaranjini, B.; Mangaiyarkarasi, R.; Ganesh, V.; Umadevi, S. Vertical alignment of liquid crystals over a functionalized flexible substrate. Sci. Rep. 2018, 8, 1–13. [Google Scholar]
- Ishihara, S.; Mizusaki, M. Alignment control technology of liquid crystal molecules. J. Soc. Inf. Disp. 2020, 28, 44–74. [Google Scholar]
- Kawatsuki, N.; Matsuyoshi, K.; Hayashi, M.; Takatsuka, H.; Yamamoto, T. Photoreaction of photo-cross-linkable methacrylate polymer films comprising 2-cinnamoyloxyethoxybiphenyl side group by linearly polarized ultraviolet light and liquid crystal alignment on the resultant films. Chem. Mater. 2000, 12, 1549–1555. [Google Scholar]
- Rempel, T.D.; Gandy, R.F.; Wootton, A.J. Density fluctuation effects on electron cyclotron emission correlation measurements in optically gray plasmas. Rev. Sci. Instrum. 1994, 65, 2044–2048. [Google Scholar]
- Van Aerle, N.; Tol, A. Molecular orientation in rubbed polyimide alignment layers used for liquid-crystal displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar]
- Park, H.; Lee, J.; Dong, K.; Oh, B.; Kim, Y.; Jeong, H.; Ju, B.; Seo, D. Homeotropic alignment of liquid crystals on a nano-patterned polyimide surface using nanoimprint lithography. Soft Matter 2011, 7, 5610–5614. [Google Scholar]
- Kang, D.; Kim, S.; Kim, B.; Kim, J.; Ok, C.; Kim, Y.; Han, J.; Kim, J.; Hwang, J.; Oh, B. Liquid crystal alignment effects for nematic liquid crystal on homeotropic polyimide surface using new ion-beam source. Jpn. J. Appl. Phys. 2007, 46, 6601. [Google Scholar]
- Chae, B.; Lee, S.W.; Ree, M.; Jung, Y.M.; Kim, S.B. Photoreaction and molecular reorientation in a nanoscaled film of poly(methyl 4-(methacryloyloxy)cinnamate) studied by two-dimensional FTIR and UV correlation spectroscopy. Langmuir 2003, 19, 687–695. [Google Scholar]
- Kim, J.B.; Kim, K.C.; Ahn, H.J.; Hwang, B.H.; Hyun, D.C.; Baik, H.K. Variable liquid crystal pretilt angles on various compositions of alignment layers. Appl. Phys. Lett. 2007, 90, 043515. [Google Scholar]
- Ishihara, S.; Wakemoto, H.; Nakazima, K.; Matsuo, Y. The effect of rubbed polymer films on the liquid crystal alignment. Liq. Cryst. 1989, 4, 669–675. [Google Scholar]
- Stöhr, J.; Samant, M.G. Liquid crystal alignment by rubbed polymer surfaces: A microscopic bond orientation model. J. Electron Spectrosc. Relat. Phenom. 1999, 98, 189–207. [Google Scholar]
- Liaw, D.; Wang, K.; Huang, Y.; Lee, K.; Lai, J.; Ha, C. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar]
- Pattison, L.R.; Hexemer, A.; Kramer, E.J.; Krishnan, S.; Petroff, P.M.; Fischer, D.A. Probing the ordering of semiconducting fluorene-thiophene copolymer surfaces on rubbed polyimide substrates by near-edge x-ray absorption fine structure. Macromolecules 2006, 39, 2225–2231. [Google Scholar]
- Wu, W.; Wang, C.; Fuh, A.Y. Controlling pre-tilt angles of liquid crystal using mixed polyimide alignment layer. Opt. Express 2008, 16, 17131–17137. [Google Scholar]
- Li, H.; Liu, J.; Wang, K.; Fan, L.; Yang, S. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3,5-ditrifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. Polymer 2006, 47, 1443–1450. [Google Scholar]
- Lee, S.H.; Kim, H.Y.; Park, I.C.; Rho, B.G.; Park, J.S.; Park, H.S.; Lee, C.H. Rubbing-free, vertically aligned nematic liquid crystal display controlled by in-plane field. Appl. Phys. Lett. 1997, 71, 2851–2853. [Google Scholar]
- Bechtold, I.H.; De Santo, M.P.; Bonvent, J.; Oliveira, E.A.; Barberi, R.; Rasing, T. Rubbing-induced charge domains observed by electrostatic force microscopy: Effect on liquid crystal alignment. Liq. Cryst. 2003, 30, 591–598. [Google Scholar]
- Kim, J.; Acharya, B.R.; Kumar, S.; Ha, K.R. A method for liquid crystal alignment using in situ ultraviolet exposure during imidization of polyimide. Appl. Phys. Lett. 1998, 73, 3372–3374. [Google Scholar]
- Chigrinov, V.G.; Kozenkov, V.M.; Kwok, H. Photoalignment of Liquid Crystalline Materials: Physics and Applications, 1st ed.; John Wiley & Sons: West Sussex, UK, 2008; pp. 69–93. [Google Scholar]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar]
- O’Neill, M.; Kelly, S.M. Photoinduced surface alignment for liquid crystal displays. J. Phys. D Appl. Phys. 2000, 33, 67–84. [Google Scholar]
- Ahn, H.J.; Kim, J.B.; Kim, K.C.; Hwang, B.H.; Kim, J.T.; Baik, H.K.; Park, J.S.; Kang, D. Liquid crystal pretilt angle control using adjustable wetting properties of alignment layers. Appl. Phys. Lett. 2007, 90, 253505. [Google Scholar]
- Lee, Y.J.; Kim, Y.W.; Ha, J.D.; Oh, J.M.; Yi, M.H. Synthesis and characterization of novel polyimides with 1-octadecyl side chains for liquid crystal alignment layers. Polym. Adv. Technol. 2007, 18, 226–234. [Google Scholar]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Reea, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liquid Cryst. 2000, 349, 279–282. [Google Scholar]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I.; Park, S.; Jung, J.C.; Lee, K.H.; Ree, M. Rubbing-induced surface morphology and polymer segmental reorientations of a model brush polyimide and interactions with liquid crystals at the surface. Chem. Mater. 2003, 15, 3105–3112. [Google Scholar]
- Lee, S.B.; Shin, G.J.; Chi, J.H.; Zin, W.; Jung, J.C.; Hahm, S.G.; Ree, M.; Chang, T. Synthesis, characterization and liquid-crystal-aligning properties of novel aromatic polypyromellitimides bearing (n-alkyloxy)biphenyloxy side chains. Polymer 2006, 47, 6606–6621. [Google Scholar]
- Ju, C.; Kim, T.; Kang, H. Liquid crystal alignment behaviors on capsaicin substituted polystyrene films. RSC Adv. 2017, 7, 41376–41383. [Google Scholar]
- Ju, C.; Kim, T.; Kang, H. Renewable, eugenol—Modified polystyrene layer for liquid crystal orientation. Polymers 2018, 10, 201. [Google Scholar]
- Ju, C.; Park, C.; Kim, T.; Kang, H. Vertical alignment of liquid crystals on plant-based vanillin derivative-substituted polystyrene films. RSC Adv. 2019, 9, 14188–14193. [Google Scholar]
- Lee, K.; Paek, S.; Lien, A.; Durning, C.; Fukuro, H. Microscopic molecular reorientation of alignment layer polymer surfaces induced by rubbing and its effects on LC pretilt angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J.-C. Liquid crystal alignment property of n-alkylthiomethyl- or n-alkylsulfonylmethyl-substituted polystyrenes. Polym. Adv. Technol. 2009, 20, 878–886. [Google Scholar]
- Hanemann, T.; Haase, W. Crystal structure of 4′-pentyl-4-cyanobiphenyl (5CB). Liq. Cryst. 1995, 19, 699–702. [Google Scholar]
- Bogi, A.; Faetti, S. Elastic, dielectric and optical constants of 4′-pentyl-4-cyanobiphenyl. Liq. Cryst. 2001, 28, 729–739. [Google Scholar]
- Maze, C. Determination of nematic liquid crystal elastic and dielectric properties from the shape of a capacitance-voltage curve. Mol. Cryst. Liq. Cryst. 1978, 48, 273–287. [Google Scholar]
- Schell, K.T.; Porter, R.S. Dielectric studies of highly polar nematic liquid crystals and their mixtures. Liq. Cryst. 1990, 188, 97–103. [Google Scholar]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar]
- Royall, P.G.; Craig, D.Q.; Doherty, C. Characterisation of the glass transition of an amorphous drug using modulated DSC. Pharm. Res. 1998, 15, 1117–1121. [Google Scholar]
- Hutchinson, J. Determination of the glass transition temperature: Methods correlation and structural heterogeneity. J. Therm. Anal. 2009, 98, 579–589. [Google Scholar]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 5, 318–321. [Google Scholar]
- Wesslen, B.; Lenz, R.W.; MacKnight, W.J.; Karasz, F.E. Glass transition temperatures of poly(ethyl a-chloroacrylates). Macromolecules 1971, 4, 24–26. [Google Scholar]
- Lee, J.-C.; Litt, M.H.; Rogers, C.E. Oxyalkylene polymers with alkylsulfonylmethyl side chains: Gas barrier properties. J. Polym. Sci. Pt. B-Polym. Phys. 1998, 36, 75–83. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 129–187. [Google Scholar]
- Senta, R.; Leo, M. Glass transitions of the poly-(n-alkyl methacrylates). J. Phys. Chem. 1957, 61, 985–991. [Google Scholar]
- Kahn, F.J.; Taylor, G.N.; Schonhorn, H. Surface-produced alignment of liquid crystals. Proc. IEEE 1973, 61, 823–828. [Google Scholar]
- Kim, S.I.; Ree, M.; Shin, T.J.; Jung, J.C. Synthesis of new aromatic polyimides with various side chains containing a biphenyl mesogen unit and their abilities to control liquid-crystal alignments on the rubbed surface. J. Polym. Sci. Pol. Chem. 1999, 37, 2909–2921. [Google Scholar]
- Schwartz, J.J.; Mendoza, A.M.; Wattanatorn, N.; Zhao, Y.; Nguyen, V.T.; Spokoyny, A.M.; Mirkin, C.A.; Baše, T.; Weiss, P.S. Surface dipole control of liquid crystal alignment. J. Am. Chem. Soc. 2016, 138, 5957–5967. [Google Scholar]
- Bouchiat, M.A.; Langevin-Cruchon, D. Molecular order at the free surface of a nematic liquid crystal from light reflectivity measurements. Phys. Lett. A 1971, 34, 331–332. [Google Scholar]
- Haller, I. Alignment and wetting properties of nematic liquids. Appl. Phys. Lett. 1974, 24, 349–351. [Google Scholar]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar]
- Birdi, K.S. Surface Chemistry Essentials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 137–161. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 347–378. [Google Scholar]







| Polymer Designation | Water Contact Angle (°) a | Vertical LC Aligning Ability b |
|---|---|---|
| PCMS | 71 | X |
| PECH5 | 77 | X |
| PECH10 | 80 | ∆ |
| PECH15 | 81 | O |
| PECH20 | 82 | O |
| PECH40 | 83 | O |
| PECH60 | 85 | O |
| PECH80 | 90 | O |
| PECH | 94 | O |
| PPCH | 101 | O |
| PBCH | 101 | O |
| PAmCH | 105 | O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, K.; Kang, H. Vertical Orientation of Liquid Crystal on Comb-Like 4-(trans-4-alkylcyclohexyl)phenoxymethyl-substituted Polystyrene Containing Liquid Crystal Precursor. Polymers 2021, 13, 1404. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091404
Seo K, Kang H. Vertical Orientation of Liquid Crystal on Comb-Like 4-(trans-4-alkylcyclohexyl)phenoxymethyl-substituted Polystyrene Containing Liquid Crystal Precursor. Polymers. 2021; 13(9):1404. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091404
Chicago/Turabian StyleSeo, Kyutae, and Hyo Kang. 2021. "Vertical Orientation of Liquid Crystal on Comb-Like 4-(trans-4-alkylcyclohexyl)phenoxymethyl-substituted Polystyrene Containing Liquid Crystal Precursor" Polymers 13, no. 9: 1404. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13091404