c-Perpendicular Orientation of Poly(ʟ-lactide) Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Characterizations:
3. Results and Discussion
3.1. c⊥ Orientation of Co-Crystalline and α Phases in PLLA Films
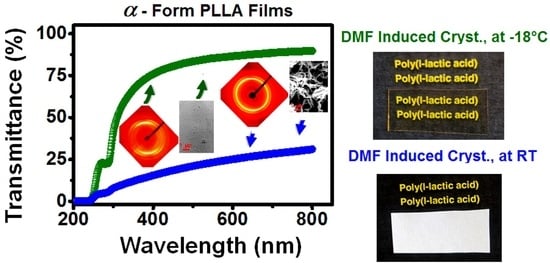
3.2. High Transparency of PLLA Films as Obtained by Low Temperature Co-Crystallization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, P.; Lamberti, M.; Albunia, A.R.; Ruiz de Ballesteros, O.; Guerra, G. Crystalline orientation in syndiotactic polystyrene cast films. Macromolecules 2002, 35, 5854–5860. [Google Scholar] [CrossRef]
- Rizzo, P.; Costabile, A.; Guerra, G. Perpendicular orientation of host polymer chains in clathrate thick films. Macromolecules 2004, 37, 3071–3076. [Google Scholar] [CrossRef]
- Rizzo, P.; Della, G.S.; Guerra, G. Perpendicular Chain Axis Orientation in s-PS Films: Achievement by Guest-Induced Clathrate Formation and Maintenance after Transitions toward Helical and Trans-Planar Polymorphic Forms. Macromolecules 2004, 37, 8043–8049. [Google Scholar] [CrossRef]
- Rizzo, P.; Spatola, A.; De Girolamo, D.M.A.; Guerra, G. Polymeric Films with Three Different Uniplanar Crystalline Phase Orientations. Macromolecules 2005, 38, 10089–10094. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Tarallo, O.; Petraccone, V.; Guerra, G. Layers of close-packed alternated enantiomorphous helices and the three different uniplanar orientations of syndiotactic polystyrene. Macromolecules 2008, 41, 8632–8642. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G. Polymeric Films with Three Different Orientations of Crystalline-Phase Empty Channels. Chem. Mater. 2009, 21, 3370–3375. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G. Two Different Uniplanar-Axial Orientations of Syndiotactic Polystyrene Films. Macromolecules 2011, 44, 5671–5681. [Google Scholar] [CrossRef]
- Rizzo, P.; Ianniello, G.; Longo, S.; Guerra, G. Uniplanar Orientations and Guest Exchange in PPO Cocrystalline Films. Macromolecules 2013, 46, 3995–4001. [Google Scholar] [CrossRef]
- Rizzo, P.; Gallo, C.; Vitale, V.; Tarallo, O.; Guerra, G. Nanoporous-crystalline films of PPO with parallel and perpendicular polymer chain orientations. Polymer 2019, 167, 193–201. [Google Scholar] [CrossRef]
- Guerra, G.; Daniel, C.; Rizzo, P.; Tarallo, O. Advanced materials based on polymer cocrystalline forms. J. Polym. Sci. Pol. Phys. 2012, 50, 305–322. [Google Scholar] [CrossRef]
- Venditto, V.; De, G.D.M.A.; Mensitieri, G.; Milano, G.; Musto, P.; Rizzo, P.; Guerra, G. Anisotropic Guest Diffusion in the δ Crystalline Host Phase of Syndiotactic Polystyrene: Transport Kinetics in Films with Three Different Uniplanar Orientations of the Host Phase. Chem. Mater. 2006, 18, 2205–2210. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G. Control of guest transport in polymer films by structure and orientation of nanoporous-crystalline phases. Polymer 2013, 54, 1671–1678. [Google Scholar] [CrossRef]
- Itagaki, H.; Sago, T.; Uematsu, M.; Yoshioka, G.; Correa, A.; Venditto, V.; Guerra, G. Guest Orientation in Uniplanar-Axial Polymer Host Films and in Co-Crystal Unit-Cell, Determined by Angular Distributions of Polarized Guest Fluorescence. Macromolecules 2008, 41, 9156–9164. [Google Scholar] [CrossRef]
- Tarallo, O.; Petraccone, V.; Daniel, C.; Guerra, G. Dipolar guest orientation in polymer co-crystals and macroscopic films. CrystEngComm 2009, 11, 2381–2390. [Google Scholar] [CrossRef]
- Daniel, C.; Rufolo, C.; Bobba, F.; Scarfato, A.; Cucolo, A.M.; Guerra, G. Ferroelectric co-crystalline polymers. J. Mater. Chem. 2011, 21, 19074–19079. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Guerra, G.; Torres, F.J.; Civalleri, B.; Zicovich-Wilson, C.M. Uniplanar Orientations as a Tool to Assign Vibrational Modes of Polymer Chain. Macromolecules 2007, 40, 3895–3897. [Google Scholar] [CrossRef]
- Rizzo, P.; Albunia, A.R.; Guerra, G. Syndiotactic Polystyrene Films with Different Uniplanar Orientations: Additional Information on Crystal Phase Transitions. Macromol. Chem. Phys. 2013, 214, 41–45. [Google Scholar] [CrossRef]
- Rizzo, P.; Ianniello, G.; Venditto, V.; Tarallo, O.; Guerra, G. Poly(L-lactic acid): Uniplanar Orientation in Cocrystalline Films and Structure of the Cocrystalline Form with Cyclopentanone. Macromolecules 2015, 48, 7513–7520. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Ten Brinke, G.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Aleman, C.; Lotz, B.; Puiggali, J. Crystal Structure of the R-Form of Poly(L-lactide). Macromolecules 2001, 34, 4795–4801. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K.; Hanesaka, M.; Ohhara, T.; Kurihara, K.; Kuroki, R.; Tamada, T.; Ozeki, T.; Kanamoto, T. Crystal Structure Analysis of Poly(l-lactic Acid) α Form On the basis of the 2-Dimensional Wide-Angle Synchrotron X-ray and Neutron Diffraction Measurements. Macromolecules 2011, 44, 6441–6452. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Complex Crystal Formation of Poly(l-lactide) with Solvent Molecules. Macromolecules 2012, 45, 1384–1397. [Google Scholar] [CrossRef]
- Matsuda, Y.; Fukatsu, A.; Tasaka, S. Solvent Exchange and Gelation Mechanism of Poly(L-lactic acid) Gel Formed by Complex Crystallization with Solvents. Chem. Lett. 2013, 42, 1046–1047. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Guest-Induced Crystal-to-Crystal Transitions of Poly(L-lactide) Complexes. J. Phys. Chem. B 2013, 117, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Shaiju, P.; Murthy, N.S.; Bhoje Gowd, E. Molecular, Crystalline, and Lamellar Length-Scale Changes in the Poly(L-lactide) (PLLA) during Cyclopentanone (CPO) Desorption in PLLA/CPO Cocrystals. Macromolecules 2016, 49, 224–233. [Google Scholar] [CrossRef]
- Jianming, Z.; Yongxin, D.; Harumi, S.; Hideto, T.; Isao, N.; Shouke, Y.; Yukihiro, O. Crystal Modifications and Thermal Behavior of Poly(L-lactic acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar]
- Jianming, Z.; Kohji, T.; Hideto, T.; Abraham, J.D. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(L-lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar]
- Macauley, N.J.; Harkin-Jones, E.M.A.; Murphy, W.R. The influence of nucleating agents on the extrusion and thermoforming of polypropylene. Polym. Eng. Sci. 1998, 38, 662–670. [Google Scholar] [CrossRef]
- Gahleitner, M.; Jääskeläinen, P.; Ratajski, E.; Paulik, C.; Reussner, J.; Wolfschwenger, J.; Neißl, W. Propylene–ethylene random copolymers: Comonomer effects on crystallinity and application properties. J. Appl. Polym. Sci. 2005, 95, 1073–1081. [Google Scholar] [CrossRef]
- Daniel, C.; Avallone, A.; Guerra, G. Syndiotactic Polystyrene Physical Gels: Guest Influence on Structural Order in Molecular Complex Domains and Gel Transparency. Macromolecules 2006, 39, 7578–7582. [Google Scholar] [CrossRef]
- Bernland, K.; Tervoort, T.; Smith, P. Phase behavior and optical- and mechanical properties of the binary system isotactic polypropylene and the nucleating/clarifying agent 1,2,3-trideoxy-4,6:5,7-bis-O-[(4-propylphenyl) methylene]-nonitol. Polymer 2009, 50, 2460–2464. [Google Scholar] [CrossRef]
- Gahleitner, M.; Grein, C.; Blell, R.; Wolfschwenger, J.; Koch, T.; Ingolic, E. Sterilization of propylene/ethylene random copolymers: Annealing effects on crystalline structure and transparency as influenced by polymer structure and nucleation. Express Polym. Lett. 2011, 5, 788–798. [Google Scholar] [CrossRef]
- Hirota, S.; Sato, T.; Tominaga, Y.; Asai, S.; Sumita, M. The effect of high-pressure carbon dioxide treatment on the crystallization behavior and mechanical properties of poly(L-lactic acid)/poly(methyl methacrylate) blends. Polymer 2006, 47, 3954–3960. [Google Scholar] [CrossRef]
- Marubayashi, H.; Akaishi, S.; Akasaka, S.; Asai, S.; Sumita, M. Crystalline Structure and Morphology of Poly(L-lactide) Formed under High-Pressure CO2. Macromolecules 2008, 41, 9192–9203. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Liao, H.-W.; Chen, J.-K.; Lee, D.-J.; Xin, Z. New transparent poly(L-lactide acid) films as high-performance bio-based nanocomposites. RSC Adv. 2016, 6, 23949–23955. [Google Scholar] [CrossRef]
- Shirahase, T.; Kikuchi, M.; Shinohara, T.; Kobayashi, M.; Takahara, A. Effect of nanoparticle SiO2 grafted poly (methyl methacrylate) on poly(L-lactic) acid crystallization. Polym. Bull. 2015, 72, 1247–1263. [Google Scholar] [CrossRef]
- Bai, D.; Liu, H.; Bai, H.; Zhang, Q.; Fu, Q. Powder metallurgy inspired low-temperature fabrication of high-performance stereocomplexed polylactide products with good optical transparency. Sci. Rep. 2016, 6, 20260. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiang, F.; Qi, X.; Yang, W.; Huang, R.; Fu, Q. Optically transparent poly(methyl methacrylate) with largely enhanced mechanical and shape memory properties via in-situ formation of polylactide stereocomplex in the matrix. Polymer 2017, 126, 231–239. [Google Scholar] [CrossRef]
- Bai, D.; Liu, H.; Bai, H.; Zhang, Q.; Fu, Q. Low-Temperature Sintering of Stereocomplex-Type Polylactide Nascent Powder: Effect of Crystallinity. Macromolecules 2017, 50, 7611–7619. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening Polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]





| Films | Density (g/cm3) (±0.04) | χd (%) (±3%) | (%) of Transmittance (At 600 nm) for 100 μm Films | (%) of Transmittance (At 600 nm) for 25 μm Films |
|---|---|---|---|---|
| Amorphous | 1.203 | 0 | ~91% | ~91% |
| DMF-induced crystallization at −18 °C | 1.229 | 35 | ~86% | ~88% |
| Cold crystallization | 1.225 | 28 | ~40% | ~55% |
| DMF-induced crystallization at room temperature | 1.234 | 41 | 0 | ~25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagendra, B.; Rizzo, P.; Daniel, C.; Baldino, L.; Guerra, G. c-Perpendicular Orientation of Poly(ʟ-lactide) Films. Polymers 2021, 13, 1572. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101572
Nagendra B, Rizzo P, Daniel C, Baldino L, Guerra G. c-Perpendicular Orientation of Poly(ʟ-lactide) Films. Polymers. 2021; 13(10):1572. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101572
Chicago/Turabian StyleNagendra, Baku, Paola Rizzo, Christophe Daniel, Lucia Baldino, and Gaetano Guerra. 2021. "c-Perpendicular Orientation of Poly(ʟ-lactide) Films" Polymers 13, no. 10: 1572. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101572