Analysis of Polyhydroxyalkanoates Granules in Haloferax mediterranei by Double-Fluorescence Staining with Nile Red and SYBR Green by Confocal Fluorescence Microscopy
Abstract
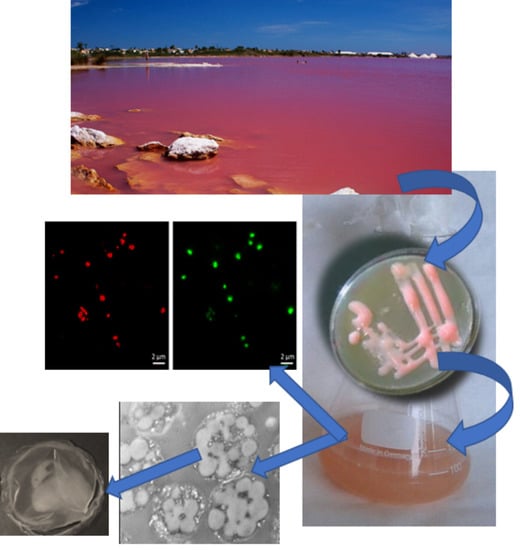
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Sample Preparation and Fluorescence Staining
2.3. Confocal Fluorescence Microscopy
2.4. Transmission Electron Microscopy (TEM)
2.5. Determination of Cell Dry Weight (CDW) and PHBV Extraction and Purification
2.6. Attenuated Total Reflectance–Fourier Transform Infrared (ATR–FTIR) Analysis
2.7. Statistics
3. Results
3.1. Nile Red Staining and Detection of PHA Granules by Confocal Microscopy
3.2. Analysis of PHA Granules Size
3.3. PHA Extraction and Polymer Characterization by ATR–FTIR Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018, 36, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Obruca, S.; Pernicova, I.; Braunegg, G. Physiological, kinetic, and process engineering aspects of polyhydroxyalkanoate biosynthesis by extremophiles. Polyhydroxyalkanoates Biosynth. Chem. Struct. Appl. 2018, 2013, 1–70. [Google Scholar]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Oren, A. Industrial and environmental applications of halophilic microorganisms. Environ. Technol. 2010, 31, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.M.R. Archaea Biotechnology. Biotechnol. Adv. 2020. [Google Scholar] [CrossRef]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal carotenoids: Healthy novel compounds from extreme environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.B.; Sprott, G.D. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef] [PubMed]
- DasSarma, S.; DasSarma, P. Halophiles and their enzymes: Negativity put to good use. Curr. Opin. Microbiol. 2015, 25, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the polyhydroxyalkanoate PHBV from ricotta cheese exhausted whey by Haloferax mediterranei fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and Characterization of Polyhydroxyalkanoates with Controlled Composition and Microstructure. Biomacromolecules 2018, 19, 996–1005. [Google Scholar] [CrossRef]
- Alsafadi, D.; Ibrahim, M.I.; Alamry, K.A.; Hussein, M.A.; Mansour, A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total Environ. 2020, 737, 139716. [Google Scholar] [CrossRef]
- Simó-Cabrera, L.; García-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Agüero, A.; Monzó Sánchez, F.; Cánovas, V.; et al. Haloarchaea as Cell Factories to Produce Bioplastics. Mar. Drugs 2021, 19. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R. Production of Polyhydroxyalkanoates (PHA) by Haloferax mediterranei from food waste derived nutrients for biodegradable plastic applications. J. Microbiol. Biotechnol. 2021, 31, 338–347. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current Developments on Polyhydroxyalkanoates Synthesis by Using Halophiles as a Promising Cell Factory. Microb. Cell Fact. 2020, 19, 1–30. [Google Scholar] [CrossRef]
- Koller, M. Polyhydroxyalkanoate biosynthesis at the edge of water activitiy-haloarchaea as biopolyester factories. Bioengineering 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Han, J.; Zhou, L.; Zhou, J.; Xiang, H. Genetic and Biochemical Characterization of the Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Synthase in Haloferax mediterranei. J. Bacteriol. 2008, 190, 4173–4180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Cai, L.; Liu, H.; Liu, X.; Han, J.; Zhou, J.; Xiang, H. Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl. Environ. Microbiol. 2012, 78, 1946–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Koller, M.; Rodríguez-Contreras, A. Techniques for tracing PHA-producing organisms and for qualitative and quantitative analysis of intra-and extracellular PHA. Life Sci. 2015, 15, 558–581. [Google Scholar] [CrossRef]
- Wei, Y.H.; Chen, W.C.; Huang, C.K.; Wu, H.S.; Sun, Y.M.; Lo, C.W.; Janarthanan, O.M. Screening and evaluation of polyhydroxybutyrate-producing strains from indigenous isolate Cupriavidus taiwanensis strains. Int. J. Mol. Sci. 2011, 12, 252–265. [Google Scholar] [CrossRef]
- Spiekermann, P.; Rehm, B.H.A.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ostle, A.G.; Holt, J.G. Nile Blue A as a Fluorescent Stain for Poly-3-Hydroxybutyrate. Appl. Environ. Microbiol. 1982, 44, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Juengert, J.; Bresan, S.; Jendrossek, D. Determination of Polyhydroxybutyrate (PHB) Content in Ralstonia eutropha Using Gas Chromatography and Nile Red Staining. Bio-Protocol. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Atlić, A.; Dias, M.; Reiterer, A.; Braunegg, G. Microbial PHA Production from Waste Raw Materials. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 85–119. [Google Scholar]
- Elain, A.; Le Fellic, M.; Corre, Y.M.; Le Grand, A.; Le Tilly, V.; Audic, J.L.; Bruzaud, S. Rapid and qualitative fluorescence-based method for the assessment of PHA production in marine bacteria during batch culture. World J. Microbiol. Biotechnol. 2015, 31, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, D.P.; Amaral, A.L.; Leal, C.; Oehmen, A.; Reis, M.A.M.; Ferreira, E.C. Polyhydroxyalkanoate granules quantification in mixed microbial cultures using image analysis: Sudan Black B versus Nile Blue A staining. Anal. Chim. Acta 2015, 865, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem. A Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef] [PubMed]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Mahansaria, R.; Choudhury, J.D.; Mukherjee, J. Polymerase chain reaction-based screening method applicable universally to environmental haloarchaea and halobacteria for identifying polyhydroxyalkanoate producers among them. Extremophiles 2015, 19, 1041–1054. [Google Scholar] [CrossRef]
- Rodrigues-Oliveira, T.; Belmok, A.; Vasconcellos, D.; Schuster, B.; Kyaw, C.M. Archaeal S-layers: Overview and current state of the art. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprott, G.D. Structures of archaebacterial membrane lipids. J. Bioenerg. Biomembr. 1992, 24, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.G.L.; de Wit, J.G.; Driessen, A.J.M.; Konings, W.N. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. BBA-Biomembr. 1994, 1193, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Singer, V.L.; Lawlor, T.E.; Yue, S. Comparison of SYBR® Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test). Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1999, 439, 37–47. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Sass, H. Sensitive Determination of Microbial Growth by Nucleic Acid Staining in Aqueous Suspension. Appl. Environ. Microbiol. 2006, 72, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Valera, F.; Ruiz-Berraquero, F.; Ramos-Cormenzana, A. Behaviour of mixed populations of halophilic bacteria in continuous cultures. Can. J. Microbiol. 1980, 26, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of Culture Conditions on Poly(beta-Hydroxybutyric Acid) Production by Haloferax mediterranei. Appl. Environ. Microbiol. 1990, 56, 2517–2521. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Sinskey, A.J.; Stubbe, J.; Tian, J.; He, A.; Lawrence, A.; Liu, P.; Watson, N.; Sinskey, A.J.; Stubbe, J. Kinetic Studies of Polyhydroxybutyrate Granule Formation in Wautersia eutropha H16 by Transmission Electron Microscopy. J. Bacteriol. 2005, 187, 3814–3824. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gutiérrez, C.A.; Latisnere-Barragán, H.; García-Maldonado, J.Q.; López-Cortés, A. Screening of polyhydroxyalkanoateproducing bacteria and PhaC-encoding genes in two hypersaline microbial mats from Guerrero Negro, Baja California Sur, Mexico. PeerJ 2018, 6, e4780. [Google Scholar] [CrossRef] [Green Version]
- Balakrishna Pillai, A.; Jaya Kumar, A.; Kumarapillai, H. Enhanced production of poly(3-hydroxybutyrate) in recombinant Escherichia coli and EDTA–microwave-assisted cell lysis for polymer recovery. AMB Express 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Dai, J.; Li, G. Electrospun composites of PHBV/pearl powder for bone repairing. Prog. Nat. Sci. Mater. Int. 2015, 25, 327–333. [Google Scholar] [CrossRef]
- Don, T.M.; Chen, C.W.; Chan, T.H. Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferax mediterranei. J. Biomater. Sci. Polym. Ed. 2006, 17, 1425–1438. [Google Scholar] [CrossRef]
- Melanie, S.; Winterburn, J.B.; Devianto, H. Production of Biopolymer Polyhydroxyalkanoates (PHA) by Extreme Halophilic Marine Archaea Haloferax mediterranei in Medium with Varying Phosphorus Concentration. J. Eng. Technol. Sci. 2018, 50, 255–271. [Google Scholar] [CrossRef]
- Koller, M.; Chiellini, E.; Braunegg, G. Study on the production and re-use of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and extracellular polysaccharide by the archaeon Haloferax mediterranei strain DSM 1411. Chem. Biochem. Eng. Q. 2015, 29, 87–98. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.C.; Ma, Y.M.; Chen, G.Q. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 2020, 58, 82–93. [Google Scholar] [CrossRef]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.-M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, A.I.; Pavlovic, R.; McGivney, J.B.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. SYBR Green I: Fluorescence properties and interaction with DNA. J. Fluoresc. 2012, 22, 1189–1199. [Google Scholar] [CrossRef]





| Strain | Carbon Source | CDW (g/L) | PHA (g/L) | Yield (PHA/CDW) g g−1 |
|---|---|---|---|---|
| Haloferax mediterranei R-4 (ATCC33500) | Glucose 10g/L | 2.31 ± 0.26 | 0.198 ± 0.06 | 0.084 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cánovas, V.; Garcia-Chumillas, S.; Monzó, F.; Simó-Cabrera, L.; Fernández-Ayuso, C.; Pire, C.; Martínez-Espinosa, R.M. Analysis of Polyhydroxyalkanoates Granules in Haloferax mediterranei by Double-Fluorescence Staining with Nile Red and SYBR Green by Confocal Fluorescence Microscopy. Polymers 2021, 13, 1582. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101582
Cánovas V, Garcia-Chumillas S, Monzó F, Simó-Cabrera L, Fernández-Ayuso C, Pire C, Martínez-Espinosa RM. Analysis of Polyhydroxyalkanoates Granules in Haloferax mediterranei by Double-Fluorescence Staining with Nile Red and SYBR Green by Confocal Fluorescence Microscopy. Polymers. 2021; 13(10):1582. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101582
Chicago/Turabian StyleCánovas, Verónica, Salvador Garcia-Chumillas, Fuensanta Monzó, Lorena Simó-Cabrera, Carmen Fernández-Ayuso, Carmen Pire, and Rosa María Martínez-Espinosa. 2021. "Analysis of Polyhydroxyalkanoates Granules in Haloferax mediterranei by Double-Fluorescence Staining with Nile Red and SYBR Green by Confocal Fluorescence Microscopy" Polymers 13, no. 10: 1582. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101582