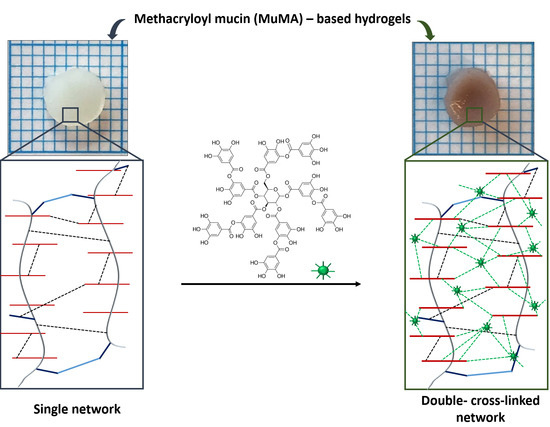
Double-Cross-Linked Networks Based on Methacryloyl Mucin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification and Characterization of Natural Macromolecules
2.1.1. Modification of Commercial Porcine Gastric Mucin
2.1.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.1.3. Circular Dichroism (CD) Spectroscopy
2.1.4. Zeta Potential
2.2. Synthesis of MuMA-Based Networks
2.3. Gel Fraction Evaluation
2.4. Swelling Ability
2.5. Mechanical Investigations
2.5.1. Uniaxial Compressions
2.5.2. Rheological Tests
2.5.3. Nanoindentation
3. Results and Discussions
3.1. Synthesis and Characterization of PGM Methacryloyl Derivative
3.2. Synthesis of the SN and DCN Systems
3.3. Assessment of the Polymerization Process
3.4. Investigation of the Water Uptake Potential of the Synthesized Systems
3.5. Evaluation of the Mechanical Behavior of the SN and DCN Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [Green Version]
- Barz, B.; Turner, B.S.; Bansil, R.; Urbanc, B. Folding of pig gastric mucin non-glycosylated domains: A discrete molecular dynamics study. J. Biol. Phys. 2012, 38, 681–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zappone, B.; Patil, N.J.; Madsen, J.B.; Pakkanen, K.I.; Lee, S. Molecular structure and equilibrium forces of bovine submaxillary mucin adsorbed at a solid-liquid interface. Langmuir 2015, 31, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Müller, M.; Rezwan, K.; Spencer, N.D. Porcine gastric mucin (PGM) at the water/poly(dimethylsiloxane) (PDMS) interface: Influence of pH and ionic strength on its conformation, adsorption, and aqueous lubrication properties. Langmuir 2005, 21, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef] [Green Version]
- Madsen, J.B.; Sotres, J.; Pakkanen, K.I.; Efler, P.; Svensson, B.; Abou Hachem, M.; Arnebrant, T.; Lee, S. Structural and mechanical properties of thin films of bovine submaxillary mucin versus porcine gastric mucin on a hydrophobic surface in aqueous solutions. Langmuir 2016, 32, 9687–9696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, J.B.; Pakkanen, K.I.; Duelund, L.; Svensson, B.; Hachem, M.A.; Lee, S. A Simplified Chromatographic Approach to Purify Commercially Available Bovine Submaxillary Mucins (BSM). Prep. Biochem. Biotechnol. 2015, 45, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; Gregor, B.; Turner, B.; Afdhal, N.H.; Bansil, R.; Erramilli, S. Viscoelastic Properties and Dynamics of Porcine Gastric Mucin. Biomacromolecules 2005, 6, 1329–1333. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Yan, X.; Lu, W.; Liu, Y. Mucin-controlled drug release from mucoadhesive phenylboronic acid-rich nanoparticles. Int. J. Pharm. 2015, 479, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Díaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.A.; Wittmann, B.; Fromme, R.; Lieleg, O. Highly Transparent Covalent Mucin Coatings Improve the Wettability and Tribology of Hydrophobic Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 28024–28033. [Google Scholar] [CrossRef]
- Olaret, E.; Ghitman, J.; Iovu, H.; Serafim, A.; Stancu, I.C. Coatings based on mucin-tannic acid assembled multilayers. Influence of pH. Polym. Adv. Technol. 2020, 31, 645–653. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. The Lubricity of Mucin Solutions Is Robust toward Changes in Physiological Conditions. ACS Appl. Bio Mater. 2019, 2, 3448–3457. [Google Scholar] [CrossRef] [Green Version]
- Janairo, R.R.R.; Zhu, Y.; Chen, T.; Li, S. Mucin covalently bonded to microfibers improves the patency of vascular grafts. Tissue Eng. Part A 2014, 20, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Co, J.Y.; Crouzier, T.; Ribbeck, K. Probing the Role of Mucin-Bound Glycans in Bacterial Repulsion by Mucin Coatings. Adv. Mater. Interfaces 2015, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Winkeljann, B.; Bauer, M.G.; Marczynski, M.; Rauh, T.; Sieber, S.A.; Lieleg, O. Covalent Mucin Coatings Form Stable Anti-Biofouling Layers on a Broad Range of Medical Polymer Materials. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Ardehali, R.; Caldwell, K.D.; Valint, P. Mucin coating on polymeric material surfaces to suppress bacterial adhesion. Colloids Surf. B Biointerfaces 2000, 17, 229–239. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [Green Version]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.Y.; Kazazian, K.; Shoichet, M.S. Peptide surface modification of methacrylamide chitosan for neural tissue engineering applications. J. Biomed. Mater. Res. A 2007, 82, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Serafim, A.; Olaret, E.; Cecoltan, S.; Butac, L.M.; Balanuca, B.; Vasile, E. Bicomponent Hydrogels Based on Methacryloyl Derivatives of Gelatin and Mucin with Potential Wound Dressing Applications. Mater. Plast. 2018, 55, 68. [Google Scholar] [CrossRef]
- Duffy, C.V.; David, L.; Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015, 20, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Serafim, A.; Cecoltan, S.; Olaret, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Mastalier, B.S.M.; Iovu, H.; Stancu, I.-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers 2020, 12, 1677. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Gong, J.P. Materials science. Materials both tough and soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, Y.; Wang, Q.; Yang, J.; Hu, S.-Q.; Chen, L. Mechanically Strong and Highly Tough Prolamin Protein Hydrogels Designed from Double-Cross-Linked Assembled Networks. ACS Appl. Polym. Mater. 2019, 1, 1272–1279. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply interpenetrating polymer networks: Preparation, mechanical properties, and applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Lafitte, G.; Kjøniksen, A.-L.; Thuresson, K.; Nyström, B. Effect of pH on the association behavior in aqueous solutions of pig gastric mucin. Carbohydr. Res. 2008, 343, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Curnutt, A.; Smith, K.; Darrow, E.; Walters, K.B. Chemical and Microstructural Characterization of pH and [Ca2+] Dependent Sol-Gel Transitions in Mucin Biopolymer. Sci. Rep. 2020, 10, 8760. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Ewoldt, R.H.; McKinley, G.H.; Bansil, R.; Erramilli, S. Rheology of Gastric Mucin Exhibits a pH-Dependent Sol−Gel Transition. Biomacromolecules 2007, 8, 1580–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and Theoretical Interpretation of Points of Zero Charge/Potential of BSA Protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Lee, S.G.; Frey, M.W. Characterizing zeta potential of functional nanofibers in a microfluidic device. J. Colloid Interface Sci. 2012, 372, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Serafim, A.; Tucureanu, C.; Petre, D.-G.; Dragusin, D.-M.; Salageanu, A.; Van Vlierberghe, S.; Dubruel, P.; Stancu, I.-C. One-pot synthesis of superabsorbent hybrid hydrogels based on methacrylamide gelatin and polyacrylamide. Effortless control of hydrogel properties through composition design. New J. Chem. 2014, 38, 3112–3126. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Ismail, O.; Doymaz, I.; Piskin, S. Modeling and investigation of the swelling kinetics of acrylamide-sodium acrylate hydrogel. J. Chem. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Balanuca, B.; Lungu, A.; Hanganu, A.M.; Stan, L.R.; Vasile, E.; Iovu, H. Hybrid nanocomposites based on POSS and networks of methacrylated camelina oil and various PEG derivatives. Eur. J. Lipid Sci. Technol. 2014, 116, 458–469. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Hanganu, A.; Lungu, A.; Iovu, H. Design of new camelina oil-based hydrophilic monomers for novel polymeric materials. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 881–891. [Google Scholar] [CrossRef]
- Argenis Caicedo, J.; Perilla, J.E. Effect of pH on the rheological response of reconstituted gastric mucin. Ing. Investig. 2015, 35, 43–48. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Ratner, B.D. Synthetic Hydrogels for Biomedical Applications—A Review. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1975, 16, 272–275. [Google Scholar]
- Nisar, S.; Pandit, A.H.; Wang, L.-F.; Rattan, S. Strategy to design a smart photocleavable and pH sensitive chitosan based hydrogel through a novel crosslinker: A potential vehicle for controlled drug delivery. RSC Adv. 2020, 10, 14694–14704. [Google Scholar] [CrossRef] [Green Version]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling Behavior of Polyacrylamide–Cellulose Nanocrystal Hydrogels: Swelling Kinetics, Temperature, and pH Effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, M.; Dabbaghi, A.; Rahmani, S. Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: Influence of network parameters on swelling and release behavior. Polym. Bull. 2020, 77, 3989–4010. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Youssefian, S.; Jakes, J.E.; Rahbar, N. Variation of Nanostructures, Molecular Interactions, and Anisotropic Elastic Moduli of Lignocellulosic Cell Walls with Moisture. Sci. Rep. 2017, 7, 2054. [Google Scholar] [CrossRef]





| Type of System | Sample Code | Dispersion Medium | pH Value | Postsynthesis Incubation |
|---|---|---|---|---|
| Single network (SN) | MuMA|MES | MES | 4.0 | DDW, 24 h |
| MuMA|PBS | PBS | 7.4 | ||
| MuMA|CB | CB | 10.0 | ||
| Double cross-linked network (DCN) | MuMA|MES_TA | MES | 4.0 | 10% TA aqueous solution, 24 h |
| MuMA|PBS_TA | PBS | 7.4 | ||
| MuMA|CB_TA | CB | 10.0 |
| Single-Network System | Gel Fraction (GF, %) |
|---|---|
| MuMA|MES | 78.63 ± 1.04 |
| MuMA|PBS | 68.31 ± 0.72 |
| MuMA|CB | 75.62 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olăreț, E.; Bălănucă, B.; Onaș, A.M.; Ghițman, J.; Iovu, H.; Stancu, I.-C.; Serafim, A. Double-Cross-Linked Networks Based on Methacryloyl Mucin. Polymers 2021, 13, 1706. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111706
Olăreț E, Bălănucă B, Onaș AM, Ghițman J, Iovu H, Stancu I-C, Serafim A. Double-Cross-Linked Networks Based on Methacryloyl Mucin. Polymers. 2021; 13(11):1706. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111706
Chicago/Turabian StyleOlăreț, Elena, Brîndușa Bălănucă, Andra Mihaela Onaș, Jana Ghițman, Horia Iovu, Izabela-Cristina Stancu, and Andrada Serafim. 2021. "Double-Cross-Linked Networks Based on Methacryloyl Mucin" Polymers 13, no. 11: 1706. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111706