Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sucrolytic Assay
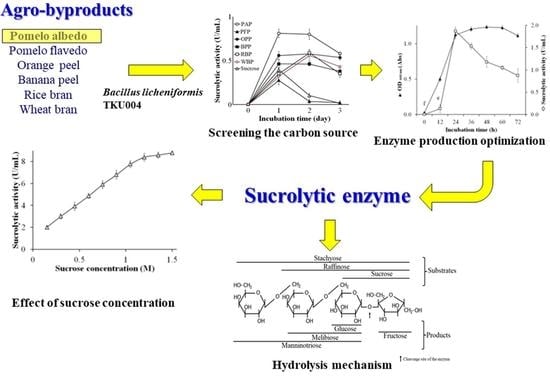
2.3. Agro-Byproducts as the Sole Carbon Source for Sucrolytic Enzymes Production
2.4. Effect of Other Conditions on Sucrolytic Enzymes Production
2.5. Enzyme Purification
2.6. Effect of Temperature and pH
2.7. Effect of Various Chemicals
2.8. Substrate Specificity
2.9. Effect of Sucrose Concentration
2.10. Statistical Analysis
3. Results and Discussion
3.1. Agro-Byproducts as the Sole Carbon Source for Sucrolytic Enzyme Production
3.2. Effect of Nitrogen Sources on Sucrolytic Enzymes Production
3.3. Effect of Phosphate Salts and Yeast Extract Powder Concentration on Sucrolytic Enzyme Production
3.4. Effect of Cultural Conditions on Sucrolytic Enzymes Production
3.5. Purification of the Produced Sucrolytic Enzyme
3.6. Identification of sleTKU004 by LC-MS/MS Analysis
3.7. Effects of Temperature and pH on the Activity and Stability of sleTKU004
3.8. Effect of Various Chemicals on the Activity of sleTKU004
3.9. Substrate Specificity of sleTKU004
3.10. Effect of Sucrose Concentration and Kinetic Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Ni, D.; Zhang, W.; Guang, C.; Zhang, T.; Mu, W. Recent advances in levansucrase and inulosucrase: Evolution, characteristics, and application. Crit. Rev. Food Sci. Nutr. 2019, 59, 3630–3647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Peng, C.; Liu, X.; Chang, F.; Xiao, Y.; Liu, J.; Fang, Z. Identification and immobilization of an invertase with high specific activity and sucrose tolerance ability of Gongronella sp. w5 for high fructose syrup preparation. Front. Microbiol. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Karboune, S.; Narwani, T.J.; de Brevern, A.G. Investigating the product profiles and structural relationships of new levansucrases with conventional and non-conventional substrates. Int. J. Mol. Sci. 2020, 21, 5402. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ spent grain as substrate for dextran biosynthesis by Leuconostoc pseudomesenteroides DSM20193 and Weissella confusa A16. Microb Cell Fact. 2021, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Meng, X.; Dijkhuizen, L.; Liu, W. Structures, physico-chemical properties, production and (potential) applications of sucrose-derived α-d-glucans synthesized by glucansucrases. Carbohydr. Polym. 2020, 249, 116818. [Google Scholar] [CrossRef] [PubMed]
- Trollope, K.M.; van Wyk, N.; Kotjomela, M.A.; Volschenk, H. Sequence and structure-based prediction of fructosyltransferase activity for functional subclassification of fungal GH32 enzymes. FEBS J. 2015, 282, 4782–4796. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, K.; Lammens, W.; Verhaest, M.; de Coninck, B.; Rabijns, A.; van Laere, A.; van den Ende, W. Unraveling the difference between invertases and fructan exohydrolases: A single amino acid (Asp-239) substitution transforms Arabidopsis cell wall invertase1 into a fructan 1-exohydrolase. Plant. Physiol. 2007, 145, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.L.; Craft, K.M.; Townsend, S.D. Infant food applications of complex carbohydrates: Structure, synthesis, and function. Carbohydr. Res. 2017, 437, 16–27. [Google Scholar] [CrossRef]
- González-Garcinuño, A.; Tabernero, A.; Domínguez, A.; Galán, M.A.; del Valle, E.M.M. Levan and levansucrases: Polymer, enzyme, micro-organisms and biomedical applications. Biocatal. Biotransform. 2018, 36, 233–244. [Google Scholar] [CrossRef]
- Taskin, M.; Ortucu, S.; Unver, Y.; Canli, O. Invertase production and molasses decolourization by cold-adapted filamentous fungus Cladosporium herbarum ER-25 in non-sterile molasses medium. Process. Saf. Environ. Protect. Instit. Chem. Eng. 2016, 103, 136–143. [Google Scholar] [CrossRef]
- Hoffmann, J.J.; Hövels, M.; Kosciow, K.; Deppenmeier, U. Synthesis of the alternative sweetener 5-ketofructose from sucrose by fructose dehydrogenase and invertase producing Gluconobacter strains. J. Biotechnol. 2020, 307, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Rasbold, L.M.; Heinen, P.R.; da Conceição Silva, J.L.; Simão, R.d.C.G.; Kadowaki, M.K.; Maller, A. Cunninghamella echinulata PA3S12MM invertase: Biochemical characterization of a promiscuous enzyme. J. Food Biochem. 2021, 45, e13654. [Google Scholar] [CrossRef]
- Contesini, F.J.; de Alencar Figueira, J.; Kawaguti, H.Y.; de Barros Fernandes, P.C.; de Oliveira Carvalho, P.; da Graça Nascimento, M.; Sato, H.H. Potential applications of carbohydrases immobilization in the food industry. Int. J. Mol. Sci. 2013, 14, 1335–1369. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A review on invertase: Its potentials and applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Lincoln, L.; More, S.S. Bacterial invertases: Occurrence, production, biochemical characterization, and significance of transfructosylation. J. Basic Microbiol. 2017, 57, 803–813. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Tyagi, P.; Yadavilli, S. Invertase and its application—A brief review. J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Mariz, B.P.; Carvalho, S.; Batalha, I.L.; Pina, A.S. Artificial enzymes bringing together computational design and directed evolution. Org. Biomol. Chem. 2021, 19, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Sehgal, S. Microbial enzymes in food processing. In Biocatalysis; Husain, Q., Ullah, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 255–272. [Google Scholar]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Wang, S.-L. Conversion of wheat bran to xylanases and dye adsorbent by Streptomyces thermocarboxydus. Polymers 2021, 13, 287. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of seafood processing by-products for production of proteases by Paenibacillus sp. TKU052 and their application in biopeptides’ preparation. Mar. Drugs 2020, 18, 574. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Chen, C.L.; Nguyen, V.B.; Tran, T.N.; Nguyen, A.D.; Wang, S.L. Conversion of wheat bran to pectinases by Bacillus amyloliquefaciens and its applications on hydrolyzing banana peels for prebiotics production. Polymers 2021, 13, 1483. [Google Scholar] [CrossRef]
- Hammami, A.; Bayoudh, A.; Abdelhedi, O.; Nasri, M. Low-cost culture medium for the production of proteases by Bacillus mojavensis SA and their potential use for the preparation of antioxidant protein hydrolysate from meat sausage by-products. Ann. Microbiol. 2018, 68, 473–484. [Google Scholar] [CrossRef]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.L. Bioprocessing of squid pens waste into chitosanase by Paenibacillus sp. TKU047 and its application in low-molecular weight chitosan oligosaccharides production. Polymers 2020, 12, 1163. [Google Scholar] [CrossRef]
- Wang, C.L.; Su, J.W.; Liang, T.W.; Nguyen, A.D.; Wang, S.L. Production, purification and characterization of a chitosanase from Bacillus cereus. Res. Chem. Intermed. 2014, 40, 2237–2248. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosaminidase-like activity from shrimp head conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Ohara, A.; de Castro, R.J.S.; Nisshde, T.G.; Dias, F.F.G.; Bagagli, M.P.; Sato, H.H. Invertase production by Asspergillus niger under solid state fermentation: Focus on physical-chemical parameters, synergistic and antagonistic effects using agro-industrial wastes. Biocatal. Agric. Biotechnol. 2015, 4, 645–652. [Google Scholar] [CrossRef]
- Clements, L.D.; Miller, B.S.; Streips, U.N. Comparative growth analysis of the facultative anaerobes Bacillus subtilis, Bacillus licheniformis, and Escherichia coli. Syst. Appl. Microbiol. 2002, 25, 284–286. [Google Scholar] [CrossRef]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef]
- Sellami-Kamoun, A.; Haddar, A.; Ne, H.A.; Ghorbel-Frikha, B.; Kanoun, S.; Nasri, M. Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol. Res. 2008, 163, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fincan, S.A.; Özdemir, S.; Karakaya, A.; Enez, B.; Mustafov, S.D.; Ulutaş, M.S.; Şen, F. Purification and characterization of thermostable α-amylase produced from Bacillus licheniformis So-B3 and its potential in hydrolyzing raw starch. Life Sci. 2021, 264, 118639. [Google Scholar] [CrossRef]
- De Boer, A.S.; Priest, F.; Diderichsen, B. On the industrial use of Bacillus licheniformis: A review. Appl. Microbiol. Biotechnol. 1994, 40, 595–598. [Google Scholar] [CrossRef]
- Nakapong, S.; Pichyangkura, R.; Ito, K.; Iizuka, M.; Pongsawasdi, P. High expression level of levansucrase from Bacillus licheniformis RN-01 and synthesis of levan nanoparticles. Int. J. Biol. Macromol. 2013, 54, 30–36. [Google Scholar] [CrossRef]
- Lu, L.; Fu, F.; Zhao, R.; Jin, L.; He, C.; Xu, L.; Xiao, M. A recombinant levansucrase from Bacillus licheniformis 8-37-0-1 catalyzes versatile transfructosylation reactions. Process. Biochem. 2014, 49, 1503–1510. [Google Scholar] [CrossRef]
- Xavier, J.R.; Ramana, K.V. Optimization of levan production by cold-active Bacillus licheniformis ANT 179 and fructooligosaccharide synthesis by its levansucrase. Appl. Biochem. Biotechnol. 2017, 181, 986–1006. [Google Scholar] [CrossRef]
- Parrado, J.; Rodriguez-Morgado, B.; Tejada, M.; Hernandez, T.; Garcia, C. Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme Microb. Technol. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Wang, S.L.; Kao, T.Y.; Wang, C.L.; Yen, Y.H.; Chern, M.K.; Chen, Y.H. A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzym. Microb. Technol. 2006, 39, 724–731. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-oxidant and anti-diabetes potential of water-soluble chitosan–glucose derivatives produced by Maillard reaction. Polymers 2019, 11, 1714. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.-W.; Lo, B.-C.; Wang, S.-L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and pigments adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zain, N.F.M.; Yusop, S.M.; Ahmad, I. Preparation and characterization of cellulose and nanocellulose from pomelo (Citrus grandis) albedo. J. Nutr. Food Sci. 2014, 5, 1000334. [Google Scholar]
- Huang, R.; Cao, M.; Guo, H.; Qi, W.; Su, R.; He, Z. Enhanced ethanol production from pomelo peel waste by integrated hydrothermal treatment, multienzyme formulation, and fed-batch operation. J. Agric. Food Chem. 2014, 62, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Guzzon, R.; Caruso, M.; Licciardello, C.; Martens, S. Alcoholic fermentation of citrus flavedo and albedo with pure and mixed yeast strains: Physicochemical characteristics and phytochemical profiles. LWT 2021, 144, 111133. [Google Scholar] [CrossRef]
- Bahena, J.M.V.; Estrada, J.V.; Hernandez, J.A.S.; Lopez, J.O. Expression and improved production of the soluble extracellular invertase from Zymomonas mobilisin in Escherichia coli. Enzyme Microb. Technol. 2006, 40, 61–66. [Google Scholar] [CrossRef]
- Warchol, M.; Perrin, S.; Grill, J.P.; Schneider, F. Characterization of a purified β-fructofuranosidase from Bifidobacterium infantis ATCC 15697. Lett. Appl. Microbiol. 2002, 35, 462–467. [Google Scholar] [CrossRef]
- Nehad, E.A.; Atalla, S.M. Production and immobilization of invertase from Penicillium sp. using orange peel waste as substrate. Egypt Pharmaceut. J. 2020, 19, 103–112. [Google Scholar]
- Do Nascimento, G.C.; Batista, R.D.; Santos, C.C.A.D.A.; da Silva, E.M.; de Paula, F.C.; Mendes, D.B.; de Oliveira, D.P.; de Almeida, A.F. β-Fructofuranosidase and β-D-Fructosyltransferase from new Aspergillus carbonarius PC-4 strain isolated from canned peach syrup: Effect of carbon and nitrogen sources on enzyme production. Sci. World J. 2019, 2019, 6956202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.B.; Nguyen, D.N.; Nguyen, A.D.; Ngo, V.A.; Ton, T.Q.; Doan, C.T.; Pham, T.P.; Tran, T.P.H.; Wang, S.-L. Utilization of crab waste for cost-effective bioproduction of prodigiosin. Mar. Drugs 2020, 18, 523. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Mohkam, M.; Ghasemian, A.; Rasoul-Amini, S. Experimental design of medium optimization for invertase production by Pichia sp. J. Food Sci. Technol. 2014, 51, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.; Choi, W.; Kwon, S.; Yi, S.; Lee, D.; Lee, J. Purification and properties of intracellulase invertase from alkalophilic and thermophilic Bacillus cereus TA-11. J. Appl. Biol. Chem. 2007, 50, 196–201. [Google Scholar]
- Ahmed, S.A. Invertase production by Bacillus macerans immobilized on calcium alginate beads. J. Appl. Sci. Res. 2008, 4, 1777–1781. [Google Scholar]
- Awad, G.E.A.; Amer, H.; Gammal, E.W.E.; Helmy, W.A. Production optimization of invertase by Lactobacillus brevis Mm-6 and its immobilization on alginate beads. Carbohydr. Polym. 2013, 93, 740–746. [Google Scholar] [CrossRef]
- Aracri, F.M.; Cavalcanti, R.M.F.; Guimaraes, L.H.S. Extracellular tannase from Aspergillus ochraceus: Influence of the culture conditions on biofilm formation, enzyme production, and application. J. Microbiol. Biotechnol. 2019, 29, 1749–1759. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, A.D. Production, optimization and characterization of extracellular invertase by an actinomycete strain. J. Sci. Ind. Res. 2005, 64, 515–519. [Google Scholar]
- Yamamoto, K.; Kitamoto, Y.; Ohata, N.; Isshiki, S. Purification and properties of invertase from a glutamate-producing bacterium. J. Ferment. Technol. 1986, 64, 285–291. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, Y.Q.; Wang, Y.H.; Yang, B. Production of β-fructofuranosidase by Arthrobacter sp. and its application in the modification of stevioside and rebaudioside A. Food Technol. Biotechnol. 2009, 47, 137–143. [Google Scholar]
- Li, Y.; Ferenci, T. The Bacillus stearothermophilus NUB36 surA gene encodes a thermophilic sucrase related to Bacillus subtilis SacA. Microbiology 1996, 142, 1651–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambert, R.; Rain-Guion, M.C.; Petit-Glatron, M.F. Readthrough of the Bacillus subtilis stop codon produces an extended enzyme displaying a higher polymerase activity. Biochim. Biophys. Acta 1992, 1132, 145–153. [Google Scholar] [CrossRef]
- Lincon, L.; More, S.S.; Reddy, S.V. Purification and biochemical characterization of β-d-fructofuranosidase from Bacillus subtilis LYN12. J. Food Biochem. 2018, 42, e12592. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, H.; Zhao, F.; Zhao, B.; Xu, M.; Zhou, Z.; Han, Y. A fructan sucrase secreted extracellular and purified in one-step by gram-positive enhancer matrix particles. Processes 2021, 9, 95. [Google Scholar] [CrossRef]
- Pascal, M.; Kunst, F.; Lepesant, J.A.; Dedonder, R. Characterization of two sucrase activities in Bacillus subtilis Marburg. Biochimie 1971, 53, 1059–1066. [Google Scholar] [CrossRef]
- Zhou, J.; He, L.; Gao, Y.; Han, N.; Zhang, R.; Wu, Q.; Li, J.; Tang, X.; Xu, B.; Ding, J.; et al. Characterization of a novel low-temperature-active, alkaline and sucrose-tolerant invertase. Sci. Rep. 2016, 6, 32081. [Google Scholar] [CrossRef] [Green Version]
- Martin, I.; Débarbouillé, M.; Ferrari, E.; Klier, A.; Rapoport, G. Characterization of the levanase gene of Bacillus subtilis which shows homology to yeast invertase. Mol. Gen. Genet. 1987, 208, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhou, Y.; Wu, X.; Montalban-Lopez, M.; Wang, L.; Li, X.; Zheng, Z. Secretion of Bacillus amyloliquefaciens levansucrase from Bacillus subtilis and its application in the enzymatic synthesis of levan. ACS Food Sci. Technol. 2021, 1, 249–259. [Google Scholar] [CrossRef]
- Schönert, S.; Buder, T.; Dahl, M.K. Identification and enzymatic characterization of the maltose-inducible alpha-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis. J. Bacteriol. 1998, 180, 2574–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phengnoi, P.; Charoenwongpaiboon, T.; Wangpaiboon, K.; Klaewkla, M.; Nakapong, S.; Visessanguan, W.; Ito, K.; Pichyangkura, R.; Kuttiyawong, K. Levansucrase from Bacillus amyloliquefaciens KK9 and its Y237S variant producing the high bioactive levan-type fructooligosaccharides. Biomolecules 2020, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Salama, B.M.; Helmy, W.A.; Ragab, T.I.M.; Ali, M.M.; Taie, H.A.A.; Esawy, M.A. Characterization of a new efficient low molecular weight Bacillus subtilis NRC 16 levansucrase and its levan. J. Basic Microbiol. 2019, 59, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, T.; Jiang, B.; Mu, W.; Miao, M. Purification and characterization of an intracellular levansucrase derived from Bacillus methylotrophicus SK 21.002. Biotechnol. Appl. Biochem. 2015, 62, 815–822. [Google Scholar] [CrossRef]
- Ammar, Y.B.; Matsubara, T.; Ito, K.; Iizuka, M.; Limpaseni, T.; Pongsawasdi, P.; Minamiura, N. Characterization of a thermostable levansucrase from Bacillus sp. TH4-2 capable of producing high molecular weight levan at high temperature. J. Biotechnol. 2002, 99, 111–119. [Google Scholar] [CrossRef]










| Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Recovery (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Cultural supernatant | 1170.91 | 582.31 | 0.497 | 100.00 | 1.00 |
| (NH4)2SO4 precipitation | 359.71 | 297.74 | 0.827 | 51.13 | 1.66 |
| Ion-exchange chromatography | 79.98 | 199.47 | 2.494 | 34.26 | 5.02 |
| Size-exclusion chromatography | 1.34 | 7.63 | 5.670 | 1.31 | 11.40 |
| Enzyme/Strain | Opt. Temp. | Opt. pH | Carbon Source | MW | Ref |
|---|---|---|---|---|---|
| Levansucrase B. licheniformis TKU004 | 50 °C | 6 | PAP | 53 kDa | This study |
| β-d-fructofuranosidase B. subtilis LYN12 | 30–60 °C | 4–8 | wheat bran and molasses | 66 kDa and 64,512.31 Da 1 | [68] |
| Levansucrase B. licheniformis RN-01 | 50 °C | 6 | peptone | 52 kDa | [42] |
| Levansucrase B. licheniformis 8-37-0-1 | 45–50 °C | 6 | sucrose | 51 kDa | [43] |
| Fructan sucrase B. subtilis ZW019 | 50 | 5.6 | tryptone | 58 kDa | [69] |
| Sucrase and levansucrase B. subtilis Marburg | 40 kDa | [70] | |||
| Invertase Bacillus sp. HJ14 | 30–32.5 °C | 8 | 58 kDa | [71] | |
| Levanase B. subtilis | sucrose | 73 kDa | [72] | ||
| Levansucrase B. amyloliquefaciens BH072 | 40 °C | 6 | sucrose and corn starch | 55 kDa | [73] |
| Invertase B. cereus TA-11 | 50 °C | 7 | sucrose | 23 kDa and 26 kDa 2 | [59] |
| Sucrase B. stearothermophilus NUB36 | 55 °C | 51,519 Da 3 105,000 Da 2 | [66] | ||
| α-glucosidase B. subtilis | 66 kDa | [74] | |||
| levansucrase B. amyloliquefaciens KK9 | 52,974 Da 3 | [75] | |||
| levansucrase B. subtilis NRC16 | 45 °C | 8.2 | starch | 14 kDa | [76] |
| Levansucrase B. methylotrophicus SK 21.002 | 40 °C | 6.5 | sucrose | 60 kDa | [77] |
| Levansucrase B. licheniformis ANT 179 | 60 °C | 6 | sugarcane juice | 25 kDa | [44] |
| Levansucrase Bacillus sp. | 60 °C | 6 | starch | 56 kDa | [78] |
| Matched Peptide Sequence | Identified Protein and Coverage Rate | Mass and pI | Strain |
|---|---|---|---|
| 39ETYGISHITR48 63YQVPEFDSSTIK74 115NADDTSIYMFYQKVGETSIDSWK137 145DSDKFDANDSILK157 177LFYTDFSGK185 216SIFDGDGKTYQNVQQFIDEGNYSSGDNHTLR246 326KVMKPLIASNTVTDEIER343 421GNNVVITSYMTNR433 461DSILEQGQLTVNK473 | Levansucrase 30% | 52,938 Da 6.18 | B. subtilis subsp. subtilis strain 168 |
| Substrate | Relative Activity (%) |
|---|---|
| Sucrose | 100.00 ± 4.44 |
| Raffinose | 85.74 ± 3.45 |
| Stachyose | 73.60 ± 5.39 |
| FOS | 19.15 ± 2.47 |
| Starch | 8.82 ± 1.93 |
| Pectin | 81.38 ± 2.60 |
| Dialyzed pectin | 15.26 ± 3.28 |
| Dextran | 16.94 ± 5.04 |
| Gum arabic | 9.96 ± 1.65 |
| 1,3-β-glucan | 3.59 ± 2.24 |
| Maltose | N.A. |
| Sucralose | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.-L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers 2021, 13, 1959. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121959
Doan CT, Tran TN, Nguyen TT, Tran TPH, Nguyen VB, Tran TD, Nguyen AD, Wang S-L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers. 2021; 13(12):1959. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121959
Chicago/Turabian StyleDoan, Chien Thang, Thi Ngoc Tran, Thi Thanh Nguyen, Thi Phuong Hanh Tran, Van Bon Nguyen, Trung Dung Tran, Anh Dzung Nguyen, and San-Lang Wang. 2021. "Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source" Polymers 13, no. 12: 1959. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121959