Structural and Rheological Properties of Nonedible Vegetable Oil-Based Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
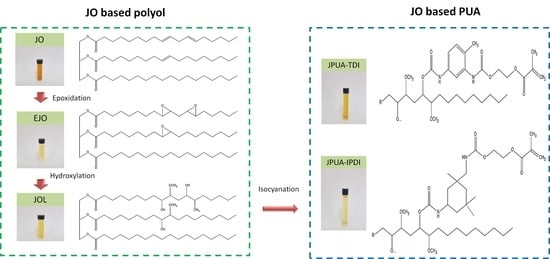
2.2. Synthesis of Jatropha Oil-Based Polyol (JOL)
2.3. Synthesis of Jatropha Oil Polyurethane Acrylate (JPUA)
2.4. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.5. Preparation of JO, JPUA-TDI, JPUA-IPDI and Their Mixtures
2.6. Rheological Properties
3. Results and Discussion
3.1. 1H NMR Analysis
3.2. Rheological Properties
3.2.1. Effect of Chemical Structure
3.2.2. Effect of Temperature
3.2.3. Development of Master Curve Graph
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, S.D.; Kurmvanshi, S.K.; Mohanty, S.; Nayak, S.K. A Review on Acrylate-Terminated Urethane Oligomers and Polymers: Synthesis and Applications. Polym. Plast. Technol. Eng. 2018, 57, 625–656. [Google Scholar] [CrossRef]
- Kunwong, D.; Sumanochitraporn, N.; Kaewpirom, S. Curing behavior of a UV-curable coating based on urethane acrylate oligomer: The influence of reactive monomers. Songklanakarin J. Sci. Technol. 2011, 33, 201–207. [Google Scholar]
- Madhi, A.; Shirkavand Hadavand, B.; Amoozadeh, A. UV-curable urethane acrylate zirconium oxide nanocomposites: Synthesis, study on viscoelastic properties and thermal behavior. J. Compos. Mater. 2018, 52, 2973–2982. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, C.; Liang, H.; Wang, Z.; Wang, H. Preparation of UV-Curable Low Surface Energy Polyurethane Acrylate/Fluorinated Siloxane Resin Resistance Properties. Materials 2020, 13, 1388. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Wang, X.; Lin, G.; Xi, L.; Yang, Y.; Lei, D.; Dong, H.; Su, J.; Cui, Y.; Liu, X. Preparation, characterization and application of UV-curable flexible hyperbranched polyurethane acrylate. Polymers 2017, 9, 552. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-curable waterborne polyurethane-acrylate: Preparation, characterization and properties. Prog. Org. Coatings 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Photo-stimulated self-healing polyurethane containing dihydroxyl coumarin derivatives. Polymers 2012, 53, 2691–2698. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Abdullah, B.M.; Yusop, R.M.; Salimon, J.; Yousif, E.; Salih, N. Physical and Chemical Properties Analysis of Jatropha curcas Seed Oil for Industrial Applications. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2013, 7, 893–896. [Google Scholar]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Synthesis of Vegetable Oil-Based Polyurethane: A Study on Curing Kinetics Behavior. Int. J. Chem. Kinet. 2016, 48, 622–634. [Google Scholar] [CrossRef]
- Ling, J.S.; Ahmed Mohammed, I.; Ghazali, A.; Khairuddean, M. Novel poly(alkyd-urethane)s from vegetable oils: Synthesis and properties. Ind. Crops Prod. 2014, 52, 74–84. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Desappan, V.; Viswanathan, J. Based Polyurethanes Using Modified Sesame Oil as Cross-Linker Containing Different NCO Groups with Improved Thermal Stability. Asian J. Chem. 2019, 31, 2375–2382. [Google Scholar] [CrossRef]
- Ahmed, W.A.; Salimon, J. Phorbol ester as toxic constituents of tropical Jatropha curcas seed oil. Eur. J. Sci. Res. 2009, 31, 429–436. [Google Scholar]
- Pandey, V.C.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R.P. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sustain. Energy Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.R.; Ho, Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011, 12, 5955–5970. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Aprilia, N.A.S.; Bhat, A.H.; Jawaid, M.; Paridah, M.T.; Rudi, D. A Jatropha biomass as renewable materials for biocomposites and its applications. Renew. Sustain. Energy Rev. 2013, 22, 667–685. [Google Scholar] [CrossRef]
- Seneviratne, K.; Jayathilaka, N. Coconut Oil: Chemistry and Nutrition; Lakva Publishers: Battaramulla, Sri Lanka, 2016; ISBN 9789551605360. [Google Scholar]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Rayung, M. Comparative study of aromatic and cycloaliphatic isocyanate effects on physico-chemical properties of bio-based polyurethane acrylate coatings. Polymers 2020, 12, 1494. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, M.Z.; Wu, Q.C.; Zhou, X.; Ge, X.W. Microencapsulation of UV-curable self-healing agent for smart anticorrosive coating. Chin. J. Chem. Phys. 2014, 27, 607–615. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Evaluation and failure analysis of linseed oil encapsulated self-healing anticorrosive coating. Prog. Org. Coat. 2018, 118, 108–115. [Google Scholar] [CrossRef]
- Es-haghi, H.; Mirabedini, S.M.; Imani, M.; Farnood, R.R. Preparation and characterization of pre-silane modified ethyl cellulose-based microcapsules containing linseed oil. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 71–80. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Ashrafi, A.; Kasiriha, M. Tung oil: An autonomous repairing agent for self-healing epoxy coatings. Prog. Org. Coat. 2011, 70, 383–387. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Wang, H.; Zhu, Y.; Wang, B. Preparation and application of polysulfone microcapsules containing tung oil in self-healing and self-lubricating epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Saman, N.M.; Ang, D.T.C.; Shahabudin, N.; Gan, S.N.; Basirun, W.J. UV-curable alkyd coating with self-healing ability. J. Coat. Technol. Res. 2018. [Google Scholar] [CrossRef]
- Shahabudin, N.; Yahya, R.; Gan, S.N. Microcapsules of Poly(urea-formaldehyde) (PUF) Containing alkyd from Palm Oil. Mater. Today Proc. 2016, 3S, S88–S95. [Google Scholar] [CrossRef]
- Shisode, P.S.; Patil, C.B.; Mahulikar, P.P. Preparation and Characterization of Microcapsules Containing Soybean Oil and Their Application in Self-Healing Anticorrosive Coatings. Polym. Plast. Technol. Eng. 2018, 57, 1334–1343. [Google Scholar] [CrossRef]
- Chiba, M.; Anetai, K.; Yamada, C.; Sato, Y.; Okuyama, H.; Sugiura, M.; Pletincx, S.; Verbruggen, H.; Hyono, A.; De Graeve, I.; et al. Development of self-healing coatings with micro capsules for corrosion protection of metal. ECS Trans. 2017, 75, 89. [Google Scholar] [CrossRef]
- Safaei, F.; Khorasani, S.N.; Rahnama, H.; Neisiany, R.E.; Koochaki, M.S. Single microcapsules containing epoxy healing agent used for development in the fabrication of cost efficient self-healing epoxy coating. Prog. Org. Coat. 2018, 114, 40–46. [Google Scholar] [CrossRef]
- Schröder, J.; Kleinhans, A.; Serfert, Y.; Drusch, S.; Schuchmann, H.P.; Gaukel, V. Viscosity ratio: A key factor for control of oil drop size distribution in effervescent atomization of oil-in-water emulsions. J. Food Eng. 2012, 111, 265–271. [Google Scholar] [CrossRef]
- Thanawala, K.; Mutneja, N.; Khanna, A.S.; Singh Raman, R.K. Development of self-healing coatings based on linseed oil as autonomous repairing agent for corrosion resistance. Materials 2014, 7, 7324–7338. [Google Scholar] [CrossRef] [Green Version]
- Tatiya, P.D.; Mahulikar, P.P.; Gite, V.V. Designing of polyamidoamine-based polyurea microcapsules containing tung oil for anticorrosive coating applications. J. Coat. Technol. Res. 2016, 13, 715–726. [Google Scholar] [CrossRef]
- Neon Gan, S.; Shahabudin, N. Applications of Microcapsules in Self-Healing Polymeric Materials. In Microencapsulation—Processes, Technologies and Industrial Applications; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Głowińska, E.; Datta, J. A mathematical model of rheological behavior of novel bio-based isocyanate-terminated polyurethane prepolymers. Ind. Crops Prod. 2014, 60, 123–129. [Google Scholar] [CrossRef]
- Steller, R.; Iwko, J. Shear Stress-Dependent Viscosity Master Curves for Practical Applications. Polym. Eng. Sci. 2020, 60, 44–54. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.; Ibrahim, N.A.; Mohd Dahlan, K.Z.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of radiation curable palm oil-based epoxy acrylate: NMR and FTIR spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [Green Version]
- Bjorn, A.; La Monja, P.S.; Karlsson, A.; Ejlertsson, J.; Svensson, B.H. Rheological Characterization. In Biogas; IntechOpen: London, UK, 2012; pp. 131–145. ISBN 978–953–51–0204–5. [Google Scholar]
- Steffe, J.F. Rheological Methods in Food Processing Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996; ISBN 0963203614. [Google Scholar]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Abril-Milán, D.; Valdés, O.; Mirabal-Gallardo, Y.; de la Torre, A.F.; Bustamante, C.; Contreras, J. Preparation of renewable bio-polyols from two species of Colliguaja for Rigid polyurethane foams. Materials 2018, 11, 2244. [Google Scholar] [CrossRef] [Green Version]
- Taib, E.R.J.; Abdullah, L.C.; Aung, M.M.; Basri, M.; Salleh, M.Z.; Saalah, S.; Mamat, S.; Chee, C.Y.; Wong, J.L. Physico-chemical characterisation of epoxy acrylate resin from jatropha seed oil. Pigment Resin Technol. 2017, 46, 485–495. [Google Scholar] [CrossRef]
- Wong, J.L.; Aung, M.M.; Lim, H.N.; Md Jamil, S.N.A. Spectroscopic Analysis of Epoxidised Jatropha Oil (EJO) and Acrylated Epoxidised Jatropha Oil (AEJO). Pertanika J. Trop. Agric. Sci. 2017, 40, 435–447. [Google Scholar]
- Kai Ling, C.; Aung, M.M.; Rayung, M.; Chuah Abdullah, L.; Lim, H.N.; Mohd Noor, I.S. Performance of Ionic Transport Properties in Vegetable Oil-Based Polyurethane Acrylate Gel Polymer Electrolyte. ACS Omega 2019, 4, 2554–2564. [Google Scholar] [CrossRef]
- Yildiz, Z.; Onen, H.A. Dual-curable PVB based adhesive formulations for cord/rubber composites: The influence of reactive diluents. Int. J. Adhes. Adhes. 2017, 78, 38–44. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Design and synthesis of bio-based UV curable PU acrylate resin from itaconic acid for coating applications. Des. Monomers Polym. 2017, 20, 269–282. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Shang, Q.; Zhou, Y. Synthesis and characterization of novel renewable castor oil-based UV-curable polyfunctional polyurethane acrylate. J. Coat. Technol. Res. 2018, 15, 77–85. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S. Colloidal stability and rheology of jatropha oil-based waterborne polyurethane (JPU) dispersion. Prog. Org. Coat. 2018, 125, 348–357. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Structure-rheology relationship of fully bio-based linear polyester polyols for polyurethanes—Synthesis and investigation. Polym. Test. 2018, 67, 110–121. [Google Scholar] [CrossRef]
- Vitz, E.; Moore, J.W.; Shorb, J.; Wendor, T.; Hahn, A. Viscosity. Solids, Liquids and Solutions. ChemPRIME; Chemical Education Digital Library (ChemEd DL). 2017, pp. 10.7.1–10.7.2. Available online: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)/10%3A_Solids_Liquids_and_Solutions (accessed on 1 July 2021).
- Shaw, M.T. Introduction to Polymer Rheology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 9780470388440. [Google Scholar]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction; IntechOpen: London, UK, 2012. [Google Scholar]
- Ji, D.; Fang, Z.; He, W.; Luo, Z.; Jiang, X.; Wang, T.; Guo, K. Polyurethane rigid foams formed from different soy-based polyols by the ring opening of epoxidised soybean oil with methanol, phenol, and cyclohexanol. Ind. Crops Prod. 2015, 74, 76–82. [Google Scholar] [CrossRef]
- Tathe, D.S.; Jagtap, R.N. Biobased reactive diluent for UV-curable urethane acrylate oligomers for wood coating. J. Coat. Technol. Res. 2014, 12, 187–196. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Fan, L.; Jiang, Y.; Cao, L.; Tang, Z.; Zhu, J. Synthesis and properties of a bio-based epoxy resin with high epoxy value and low viscosity. ChemSusChem 2014, 7, 555–562. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s−1. J. Food Process. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, S.N.; Ataei, S.; Neisiany, R.E. Microencapsulation of a coconut oil-based alkyd resin into poly(melamine–urea–formaldehyde) as shell for self-healing purposes. Prog. Org. Coat. 2017, 111, 99–106. [Google Scholar] [CrossRef]










| Fatty Acid | Amount (%) |
|---|---|
| Palmitic | 12.8 |
| Stearic | 7.3 |
| Oleic | 41.3 |
| Linoleic | 34.4 |
| Unsaturated | 2.7 |
| Code | JO (%) | JPUA-TDI (%) | JPUA-IPDI (%) | TMPTA (%) | Benzophenone (%) |
|---|---|---|---|---|---|
| JO | 100 | - | - | - | - |
| JPUA-TDI | - | 100 | - | - | - |
| JPUA-IPDI | - | - | 100 | - | - |
| JPUA-TDI mixture | - | 65 | - | 35 | 4 |
| JPUA-IPDI mixture | - | - | 65 | 35 | 4 |
| Sample | Functional Group per One Mole of Triglyceride (%) | ||
|---|---|---|---|
| Carbon Double Bond | Epoxy | Hydroxyl | |
| JO | 20.16 | - | - |
| EJO | 2.06 | 22.56 | - |
| JOL | 0.30 | 5.27 | 12.47 |
| Sample | Temperature (°C) | Linear Equation | k | n |
|---|---|---|---|---|
| JO | 25 | y = 0.9469x − 3.5035 | 0.030 | 0.947 |
| 40 | y = 0.9971x − 4.1949 | 0.015 | 0.997 | |
| 60 | y = 0.953x − 4.5343 | 0.011 | 0.953 | |
| 80 | y = 0.935x − 4.9147 | 0.007 | 0.935 | |
| JPUA-TDI mixture | 25 | y = 1.0997x − 1.075 | 0.341 | 1.100 |
| 40 | y = 0.3022x + 0.9576 | 2.605 | 0.302 | |
| 60 | y = 0.3301x + 0.4313 | 1.539 | 0.330 | |
| 80 | y = 0.4672x − 0.3607 | 0.697 | 0.467 | |
| JPUA-IPDI mixture | 25 | y = 0.9825x − 2.8722 | 0.057 | 0.983 |
| 40 | y = 0.9945x − 3.4319 | 0.032 | 0.994 | |
| 60 | y = 0.9941x − 4.0659 | 0.017 | 0.994 | |
| 80 | y = 0.9912x − 4.5459 | 0.011 | 0.991 |
| Sample | Temperature (°C) | ̇γ at 0.5 Pa*/5 Pa** (s−1) | Calculated ̇γ (s−1) | aT (̇γ/̇γ60) |
|---|---|---|---|---|
| JO * | 25 | 20.4 | 19.51 | 1.293 |
| 40 | 34.7 | 33.69 | 1.197 | |
| 60 | 57.1 | 54.87 | 0.999 | |
| 80 | 89.8 | 96.11 | 0.877 | |
| JPUA-TDI mixture ** | 25 | 8.5 | 11.49 | 0.002 |
| 40 | 18.5 | 8.65 | 0.141 | |
| 60 | 40.8 | 35.54 | 1.000 | |
| 80 | 61.2 | 67.99 | 0.165 | |
| JPUA-IPDI mixture * | 25 | 10.2 | 9.11 | 1.396 |
| 40 | 16.3 | 15.89 | 1.190 | |
| 60 | 30.6 | 30.02 | 1.000 | |
| 80 | 51.0 | 47.06 | 0.891 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Biak, D.R.A.; Tajau, R. Structural and Rheological Properties of Nonedible Vegetable Oil-Based Resin. Polymers 2021, 13, 2490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152490
Mudri NH, Abdullah LC, Aung MM, Biak DRA, Tajau R. Structural and Rheological Properties of Nonedible Vegetable Oil-Based Resin. Polymers. 2021; 13(15):2490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152490
Chicago/Turabian StyleMudri, Nurul Huda, Luqman Chuah Abdullah, Min Min Aung, Dayang Radiah Awang Biak, and Rida Tajau. 2021. "Structural and Rheological Properties of Nonedible Vegetable Oil-Based Resin" Polymers 13, no. 15: 2490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152490