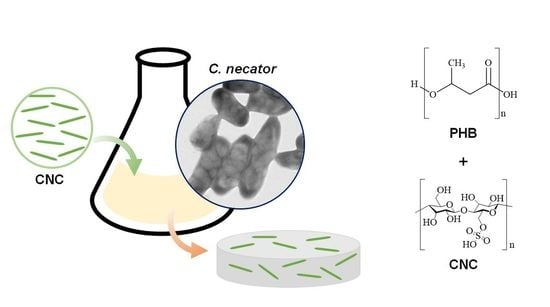
Biosynthesis of Polyhydroxybutyrate with Cellulose Nanocrystals Using Cupriavidus necator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Culture Media
2.3. Extraction of PHB
2.4. Measurement of Cell Growth
2.5. Biological Transmission Electron Microscopy
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Mechanical Properties Measurement
2.8. Water Contact Angle Measurement
2.9. Nuclear Magnetic Resonance Spectroscopy
3. Results and Discussion
3.1. Effect of CNCs on the Growth of C. necator
3.2. Characterization of the PHB–CNC Nanocomposite
3.3. Effects of CNCs Supplemented before and after Cultivation for the Nanocomposite Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Aragosa, A.; Specchia, V.; Frigione, M. Isolation of two bacterial species from argan soil in morocco associated with polyhydroxybutyrate (PHB) accumulation: Current potential and future prospects for the bio-based polymer production. Polymers 2021, 13, 1870. [Google Scholar] [CrossRef]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in drug delivery and tissue engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural Poly(3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef] [PubMed]
- S. de O. Patrício, P.; Pereira, F.V.; dos Santos, M.C.; de Souza, P.P.; Roa, J.P.B.; Orefice, R.L. Increasing the elongation at break of polyhydroxybutyrate biopolymer: Effect of cellulose nanowhiskers on mechanical and thermal properties. J. Appl. Polym. Sci. 2013, 127, 3613–3621. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.A.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.E.; Codita, I.; Dinescu, G. Poly(3-hydroxybutyrate) modified by nanocellulose and plasma treatment for packaging applications. Polymers 2018, 10, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, ultrasonic extraction, and characterization of Poly(3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- Pavan, F.A.; Junqueira, T.L.; Watanabe, M.D.B.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragao, G.M.F. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- Panich, J.; Fong, B.; Singer, S.W. Metabolic engineering of Cupriavidus necator H16 for sustainable biofuels from CO2. Trends Biotechnol. 2021, 39, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of Poly-d-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar] [PubMed]
- Tian, J.; Sinskey, A.J.; Stubbe, J. Kinetic studies of polyhydroxybutyrate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J. Bacteriol. 2005, 187, 3814–3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, L.; Cruz, M.V.; Scoma, A.; Freitas, F.; Bertin, L.; Scandola, M.; Reis, M.A.M. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int. J. Biol. Macromol. 2014, 71, 117–123. [Google Scholar] [CrossRef]
- Soto, L.R.; Byrne, E.; van Niel, E.W.J.; Sayed, M.; Villanueva, C.C.; Hatti-Kaul, R. Hydrogen and polyhydroxybutyrate production from wheat straw hydrolysate using Caldicellulosiruptor species and Ralstonia eutropha in a coupled process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef]
- Mozumder, M.S.I.; Garcia-Gonzalez, L.; De Wever, H.; Volcke, E.I. Poly(3-hydroxybutyrate) (PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Park, I.; Jho, E.H.; Nam, K. Optimization of carbon dioxide and valeric acid utilization for polyhydroxyalkanoates synthesis by Cupriavidus necator. J. Polym. Environ. 2014, 22, 244–251. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Gong, J.; Mo, L.; Li, J. A comparative study on the preparation and characterization of cellulose nanocrystals with various polymorphs. Carbohydr. Polym. 2018, 195, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, H.; Shin, G.; Lee, M.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Koo, J.M.; Eom, Y.; Park, J. Biodegradable nanocomposite of Poly(ester–co–carbonate) and cellulose nanocrystals for tough tear-resistant disposable bags. Green Chem. 2021, 23, 2293–2299. [Google Scholar] [CrossRef]
- Park, S.-A.; Eom, Y.; Jeon, H.; Koo, J.M.; Lee, E.S.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Preparation of synergistically reinforced transparent bio-polycarbonate nanocomposites with highly dispersed cellulose nanocrystals. Green Chem. 2019, 21, 5212–5221. [Google Scholar] [CrossRef]
- Kim, T.; Jeon, H.; Jegal, J.; Kim, J.H.; Yang, H.; Park, J.; Oh, D.X.; Hwang, S.Y. Trans crystallization behavior and strong reinforcement effect of cellulose nanocrystals on reinforced Poly(butylene succinate) nanocomposites. RSC Adv. 2018, 8, 15389–15398. [Google Scholar] [CrossRef] [Green Version]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of cellulose nanocrystals and bacterial cellulose on disintegrability in composting conditions of plasticized PHB nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoane, I.T.; Fortunati, E.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Development and characterization of bionanocomposites based on Poly(3-hydroxybutyrate) and cellulose nanocrystals for packaging applications. Polym. Int. 2016, 65, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, D.; Madkour, M.H.; Al-Ghamdi, M.A.; Shabbaj, I.I.; Steinbüchel, A. Large scale extraction of Poly(3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2012, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Sun, X.; Shao, Y.; Boluk, Y.; Liu, Y. The impact of cellulose nanocrystals on the aggregation and initial adhesion to a solid surface of Escherichia coli K12: Role of solution chemistry. Colloids Surf. B Biointerfaces 2015, 136, 570–576. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, G.; Jeong, D.-W.; Kim, H.; Park, S.-A.; Kim, S.; Lee, J.Y.; Hwang, S.Y.; Park, J.; Oh, D.X. Biosynthesis of Polyhydroxybutyrate with Cellulose Nanocrystals Using Cupriavidus necator. Polymers 2021, 13, 2604. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162604
Shin G, Jeong D-W, Kim H, Park S-A, Kim S, Lee JY, Hwang SY, Park J, Oh DX. Biosynthesis of Polyhydroxybutyrate with Cellulose Nanocrystals Using Cupriavidus necator. Polymers. 2021; 13(16):2604. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162604
Chicago/Turabian StyleShin, Giyoung, Da-Woon Jeong, Hyeri Kim, Seul-A Park, Semin Kim, Ju Young Lee, Sung Yeon Hwang, Jeyoung Park, and Dongyeop X. Oh. 2021. "Biosynthesis of Polyhydroxybutyrate with Cellulose Nanocrystals Using Cupriavidus necator" Polymers 13, no. 16: 2604. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162604