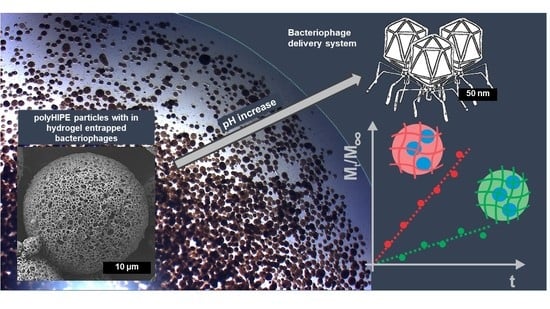
Bacteriophage Delivery Systems Based on Composite PolyHIPE/Nanocellulose Hydrogel Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteriophage and Bacterial Strains
2.3. PolyHIPE Particle Preparation
2.4. PolyHIPE Particle Characterization
2.5. Preparation of PolyHIPE/Hydrogel Particles with Encapsulated T7 Bacteriophage
2.6. Phage Titer Determination
2.7. Behavior of Phage Release from PolyHIPE/Hydrogel and the Determination of Kinetics Parameters
3. Results and Discussion
3.1. Synthesized PolyHIPE Particles
3.2. Targeted Release of Bacteriophage T7 from 1.5% TOCNF Hydrogel
3.3. Kinetics Behavior, Mechanisms, and Order of Bacteriophage Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutter, E.M.; Kuhl, S.J.; Abedon, S.T. Re-establishing a place for phage therapy in western medicine. Future Microbiol. 2015, 10, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Storms, Z.J.; Brown, T.; Cooper, D.G.; Sauvageau, D.; Leask, R.L. Impact of the cell life-cycle on bacteriophage T4 infection. FEMS Microbiol. Lett. 2014, 353, 63–68. [Google Scholar] [CrossRef]
- Abedon, S.T. Use of phage therapy to treat long-standing, persistent, or chronic bacterial infections. Adv. Drug Deliv. Rev. 2019, 145, 18–39. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górski, A.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Rogóz, P.; Jończyk-Matysiak, E.; Dabrowska, K.; Majewska, J.; Borysowski, J. Phage therapy: Combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, M.; De Sordi, L.; Debarbieux, L. The diversity of bacterial lifestyles hampers bacteriophage tenacity. Viruses 2018, 10, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Lin, H.; Ji, X.; Yan, G.; Lei, L.; Han, W.; Gu, J.; Huang, J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020, 142, 104048. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams Smith, H.; Huggins, M.B. Effectiveness of phages in treating experimental Escherichia coli diarhoea in calves, piglets and lambs. J. Gen. Microbiol. 1983, 129, 2659–2675. [Google Scholar] [CrossRef] [Green Version]
- Williams Smith, H.; Huggins, M.B.; Shaw, K.M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 1987, 133, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages-review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams Smith, H.; Huggins, M.B. Successful treatment of experimental Escherichia coli infections in mice using phage: Its general superiority over antibiotics. J. Gen. Microbiol. 1982, 128, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Johnson, C.T.; Imhoff, B.R.; Donlan, R.M.; McCarty, N.A.; García, A.J. Inhaled bacteriophage-loaded polymeric microparticles ameliorate acute lung infections. Nat. Biomed. Eng. 2018, 2, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Hornez, J.C.; Bouchart, F.; Meurice, E.; Descamps, M.; Leriche, A. Synthesis and fabrication of porous calcium phosphate ceramics for antibacterial bone substitutes. In Proceedings of the MATEC Web of Conferences, Villeneuve d’Ascq, France, 29–31 October 2013; Volume 7, pp. 10–12. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO 3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Meireles Gouvêa Boggione, D.; Boggione Santos, I.J.; de Souza, S.M.; Santos Mendonça, R.C. Preparation of polyvinyl alcohol hydrogel containing bacteriophage and its evaluation for potential use in the healing of skin wounds. J. Drug Deliv. Sci. Technol. 2021, 63, 102484. [Google Scholar] [CrossRef]
- Barros, J.A.R.; de Melo, L.D.R.; da Silva, R.A.R.; Ferraz, M.P.; de Rodrigues Azeredo, J.C.V.; de Carvalho Pinheiro, V.M.; Colaço, B.J.A.; Fernandes, M.H.R.; de Sousa Gomes, P.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102145. [Google Scholar] [CrossRef] [Green Version]
- Bosio, V.E.; Islan, G.E.; Martínez, Y.E.; Castro, G.R. Control release applications in food technology. In Advances in Bioprocesses in Food Industries; Asiatech Press: New Delhi, India, 1900; Volume 1, pp. 1–13. [Google Scholar]
- Meurice, E.; Rguiti, E.; Brutel, A.; Hornez, J.C.; Leriche, A.; Descamps, M.; Bouchart, F. New antibacterial microporous CaP materials loaded with phages for prophylactic treatment in bone surgery. J. Mater. Sci. Mater. Med. 2012, 23, 2445–2452. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Xie, Y.; Zhu, E.; Shi, Z.; Yang, Q.; Xiong, C. Doubly cross-linked nanocellulose hydrogels with excellent mechanical properties. Cellulose 2019, 26, 8645–8654. [Google Scholar] [CrossRef]
- Kopač, T.; Ručigaj, A.; Krajnc, M. The mutual effect of the crosslinker and biopolymer concentration on the desired hydrogel properties. Int. J. Biol. Macromol. 2020, 159, 557–569. [Google Scholar] [CrossRef]
- Spaic, M.; Small, D.P.; Cook, J.R.; Wan, W. Characterization of anionic and cationic functionalized bacterial cellulose nanofibres for controlled release applications. Cellulose 2014, 21, 1529–1540. [Google Scholar] [CrossRef]
- Kopač, T.; Krajnc, M.; Ručigaj, A. A mathematical model for pH-responsive ionically crosslinked TEMPO nanocellulose hydrogel design in drug delivery systems. Int. J. Biol. Macromol. 2021, 168, 695–707. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current status of alginate in drug delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Šebenik, U.; Krajnc, M.; Alič, B.; Lapasin, R. Ageing of aqueous TEMPO-oxidized nanofibrillated cellulose dispersions: A rheological study. Cellulose 2019, 26, 917–931. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, X.; Baxi, S.; Chambers, J.R.; Sabour, P.M.; Wang, Q. Whey protein improves survival and release characteristics of bacteriophage Felix O1 encapsulated in alginate microspheres. Food Res. Int. 2013, 52, 460–466. [Google Scholar] [CrossRef]
- Vinner, G.K.; Vladisavljević, G.T.; Clokie, M.R.J.; Malik, D.J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS ONE 2017, 12, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Gokmen, M.T.; Du Prez, F.E. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef] [Green Version]
- Desforges, A.; Arpontet, M.; Deleuze, H.; Mondain-Monval, O. Synthesis and functionalisation of polyHIPE® beads. React. Funct. Polym. 2002, 53, 183–192. [Google Scholar] [CrossRef]
- Kramer, S.; Cameron, N.R.; Krajnc, P. Porous polymers from high internal phase emulsions as scaffolds for biological applications. Polymers 2021, 13, 1786. [Google Scholar] [CrossRef] [PubMed]
- Huš, S.; Krajnc, P. PolyHIPEs from Methyl methacrylate: Hierarchically structured microcellular polymers with exceptional mechanical properties. Polymer 2014, 55, 4420–4424. [Google Scholar] [CrossRef]
- Štefanec, D.; Krajnc, P. 4-Vinylbenzyl chloride based porous spherical polymer supports derived from water-in-oil-in-water emulsions. React. Funct. Polym. 2005, 65, 37–45. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Van Camp, W.; Colver, P.J.; Bon, S.A.F.; Du Prez, F.E. Fabrication of Porous “Clickable” Polymer Beads and Rods through Generation of High Internal Phase Emulsion (HIPE) Droplets in a Simple Microfluidic Device. Macromolecules 2009, 42, 9289–9294. [Google Scholar] [CrossRef]
- Lapierre, F.; Cameron, N.R.; Zhu, Y. Ready… set, flow: Simple fabrication of microdroplet generators and their use in the synthesis of PolyHIPE microspheres. J. Micromech. Microeng. 2015, 25, 035011. [Google Scholar] [CrossRef] [Green Version]
- Mert, E.H.; Yıldırım, H. Porous functional poly(unsaturated polyester-co-glycidyl methacrylate-co-divinylbenzene) polyHIPE beads through w/o/w multiple emulsions: Preparation, characterization and application. E-Polymers 2014, 14, 65–73. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Q.; Shi, S.; Zhu, S. High internal phase emulsion with double emulsion morphology and their templated porous polymer systems. J. Colloid Interface Sci. 2016, 483, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.E.; Gigliobianco, G.; Sherborne, C.; Green, N.H.; Dugan, J.M.; MacNeil, S.; Reilly, G.C.; Claeyssens, F. Porous microspheres support mesenchymal progenitor cell ingrowth and stimulate angiogenesis. APL Bioeng. 2018, 2, 026103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitely, M.; Rodriguez-Rivera, G.; Waldron, C.; Mohiuddin, S.; Cereceres, S.; Sears, N.; Ray, N.; Cosgriff-Hernandez, E. Porous PolyHIPE microspheres for protein delivery from an injectable bone graft. Acta Biomater. 2019, 93, 169–179. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Green, M.R. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Mravljak, R.; Bizjak, O.; Podlogar, M.; Podgornik, A. Effect of polyHIPE porosity on its hydrodynamic properties. Polym. Test. 2021, 93, 106590. [Google Scholar] [CrossRef]
- Vinet, L.; Zhedanov, A. Bacteriophages. In Methods in Molecular Biology; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501, ISBN 978-1-58829-682-5. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Z.; Ying, X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J. Colloid Interface Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- Stroud, R.M.; Serwer, P.; Ross, M.J. Assembly of bacteriophage T7. Dimensions of the bacteriophage and its capsids. Biophys. J. 1981, 36, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Colemont, L.J.; Phillips, S.F.; Brown, M.L.; Thomforde, G.M.; Chapman, N.; Zinsmeister, A.R. Human gastric emptying and colonic filling of solids characterized by a new method. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 257, G284–G290. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Liu, H.; Ma, L.; Huang, X.; Cai, Z.; Xu, X.; Shang, S.; Song, Z. Cellulose-based polymeric emulsifier stabilized poly(N-vinylcaprolactam) hydrogel with temperature and pH responsiveness. Int. J. Biol. Macromol. 2020, 143, 190–199. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, G.; Lin, Q.; Fan, J. pH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014, 4, 47056–47065. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.K.; Prajapati, S.K.; Singh, V.K. Formulation and Evaluation of once daily sustained release matrix tablets of Aceclofenac using natural gums. J. Drug Deliv. Ther. 2012, 2. [Google Scholar] [CrossRef]
- Sutton, S. Microbiology topics. Accuracy of plate counts. J. Valid Technol. 2011, 17, 42–46. [Google Scholar]
- Sutton, S. The limitations of CFU: Compliance to CGMP requires good science. J. GXP Compliance 2012, 16, 74–80. [Google Scholar]
- Setapa, A.; Ahmad, N.; Mahali, S.M.; Amin, M.C.I.M. Mathematical model for estimating parameters of swelling drug delivery devices in a two-phase release. Polymers 2020, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, R.Y.K.; Morales, S.; Chan, H.K. Bacteriophage-delivering hydrogels: Current progress in combating antibiotic resistant bacterial infection. Antibiotics 2021, 10, 130. [Google Scholar] [CrossRef]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]





| Sample Names | |||||
|---|---|---|---|---|---|
| Biopolymer | Crosslinker (mM) | pH | k·104 (s) | n | R2 |
| 1.5% TOCNF | 15 | 7 | 3.22 | 1.09 | 0.97 |
| 0 | 7 | 5.42 | 1.04 | 0.87 | |
| 15 | 5 | 2.06 | 1.14 | 0.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopač, T.; Lisac, A.; Mravljak, R.; Ručigaj, A.; Krajnc, M.; Podgornik, A. Bacteriophage Delivery Systems Based on Composite PolyHIPE/Nanocellulose Hydrogel Particles. Polymers 2021, 13, 2648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162648
Kopač T, Lisac A, Mravljak R, Ručigaj A, Krajnc M, Podgornik A. Bacteriophage Delivery Systems Based on Composite PolyHIPE/Nanocellulose Hydrogel Particles. Polymers. 2021; 13(16):2648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162648
Chicago/Turabian StyleKopač, Tilen, Ana Lisac, Rok Mravljak, Aleš Ručigaj, Matjaž Krajnc, and Aleš Podgornik. 2021. "Bacteriophage Delivery Systems Based on Composite PolyHIPE/Nanocellulose Hydrogel Particles" Polymers 13, no. 16: 2648. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162648