Effect of Microcapsules with Waterborne Coating as Core Material on Properties of Coating for Tilia Europaea and Comparison with Other Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
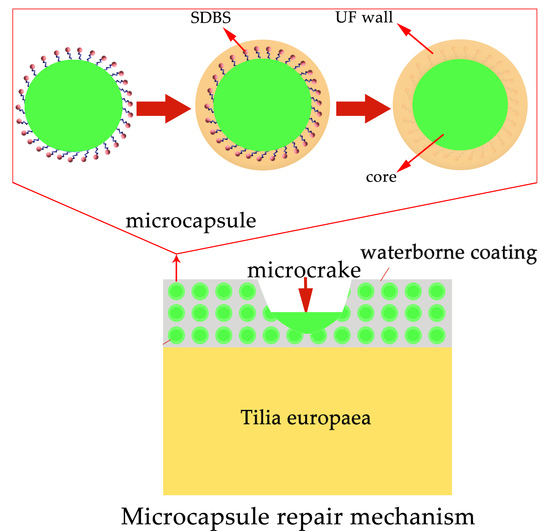
2.2. Preparation of Microcapsules
2.3. Preparation of Coating with Microcapsules
2.4. Testing and Characterization
3. Results and Discussion
3.1. Analysis of Orthogonal Test Results
3.1.1. Analysis of Micro Characteristics of Microcapsules
3.1.2. Infrared Analysis of Components in Microcapsules
3.1.3. Output Analysis of Microcapsules
3.1.4. Clad Ratio Analysis of Microcapsules
3.2. Analysis of Single Factor Test Results
3.2.1. Analysis of Micro Characteristics of Microcapsules
3.2.2. Infrared Analysis of Components in Microcapsules
3.2.3. Output Analysis of Microcapsules
3.2.4. Clad Ratio Analysis of Microcapsules
3.3. Effect of Standing Time on the Morphology of Microcapsules
3.4. Influence of Microcapsules on the Performances of Waterborne Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panek, M.; Oberhofnerova, E.; Zeidler, A.; Sedivka, P. Efficacy of Hydrophobic Coatings in Protecting Oak Wood Surfaces during Accelerated Weathering. Coatings 2017, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Rowell, R.; Bongers, F. Role of Moisture in the Failure of Coatings on Wood. Coatings 2017, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Panek, M.; Dvorak, O.; Oberhofnerova, E.; Simunkova, K.; Zeidler, A. Effectiveness of Two Different Hydrophobic Topcoats for Increasing of Durability of Exterior Coating Systems on Oak Wood. Coatings 2019, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Gao, D.; Xu, W. Influence of the Bottom Color Modification and Material Color Modification Process on the Performance of Modified Poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Lee Hia, I.; Chan, E.-S.; Chai, S.-P.; Pasbakhsh, P. A Novel Repeated Self-healing Epoxy Composite with Alginate Multicore Microcapsules. J. Mater. Chem. A 2018, 6, 8470–8478. [Google Scholar] [CrossRef]
- Gao, L.; He, J.; Hu, J.; Wang, C. Photoresponsive Self-Healing Polymer Composite with Photoabsorbing Hybrid Microcapsules. ACS Appl. Mater. Inter. 2015, 7, 25546–25552. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fang, X.; Han, J.; Wu, Z.; Zhang, J. Effect of Coating Thickness on Sound Absorption Property of Four Wood Species Commonly Used for Piano Soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Jimeno, S.; Cano-Sarabia, M.; Mejias, N.; Navarro, V.; Frances, A.B.; Maspoch, D. Healing Damaged Coatings Using Friction-sensitive Hybrid Microcapsules. J. Mater. Chem. A 2015, 3, 17966–17970. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Li, X.; Zhang, D.; Gao, J.; Du, C. A New Understanding of the Failure of Waterborne Acrylic Coatings. RSC Adv. 2017, 7, 38135–38148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Ding, C.; Pan, M.; Zhai, S. Fabrication of NCC-SiO2 Hybrid Colloids and Its Application on Waterborne Poly(Acrylic Acid) Coatings. Prog. Org. Coat. 2018, 122, 88–95. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Wu, Y.; Zuo, J.; Zhan, X. Effect of Nano-boron Carbide on the Properties of Waterborne Polyurethane Wood Coatings. J. For. Eng. 2020, 5, 181–185. [Google Scholar]
- Cai, Z.; Zhu, H.; Wang, P.; Wu, C.; Gao, W.; Mu, J.; Wei, S. Performance Optimization of UV Curable Waterborne Polyurethane Acrylate Wood Coatings Modified by Castor Oil. J. For. Eng. 2020, 5, 89–95. [Google Scholar]
- Aguirreurreta, Z.; de la Cal, J.C.; Leiza, J.R. Preparation of High Solids Content Waterborne Acrylic Coatings Using Polymerizable Surfactants to Improve Water Sensitivity. Prog. Org. Coat. 2017, 112, 200–209. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, J.; Zhao, Z.; Dong, H.; Li, Y. Study on Film Properties and VOCs of Nano-TiO2 and ZnO Modified Waterborne Paints. J. For. Eng. 2019, 4, 148–155. [Google Scholar]
- Lin, X.; Su, J.; Feng, B.; Guo, S.; Chen, Y.; Liu, P.; Wei, S. Modification of Waterborne Acrylate Coatings Using Biomass Silicon. J. For. Eng. 2019, 4, 148–154. [Google Scholar]
- Zhu, X.; Liu, Y.; Li, Z.; Wang, W. Thermochromic Microcapsules with Highly Transparent Shells Obtained through In-situ Polymerization of Urea Formaldehyde around Thermochromic Cores for Smart Wood Coatings. Sci. Rep. 2018, 8, 4015. [Google Scholar] [CrossRef] [Green Version]
- Hu, L. Development of Photochromic Wood Material by Microcapsules. BioResources 2016, 11, 9547–9559. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-G.; Jeon, J.; Seo, J.; Lee, J.-H.; Kim, S. Performance Evaluation of the Microencapsulated PCM for Wood-based Flooring Application. Energ. Convers. Manag. 2012, 64, 516–521. [Google Scholar] [CrossRef]
- Pinkl, S.; van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W.; Riegler, M. Urea-formaldehyde Microspheres as A Potential Additive to Wood Adhesive. J. Wood Sci. 2018, 64, 390–397. [Google Scholar] [CrossRef] [Green Version]
- He, S. Microwave Treatment for Enhancing the Liquid Permeability of Chinese Fir. BioResources 2014, 9, 1924–1938. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Tao, X.; Xu, W. Thermal Conductivity of Poplar Wood Veneer Impregnated with Graphene/Polyvinyl Alcohol. Forests 2021, 12, 777. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, X.; Gu, J. Synthesis of Isocyanate Microcapsules as Functional Crosslinking Agent for Wood Adhesive. J. Adhes. 2019, 97, 38–52. [Google Scholar] [CrossRef]
- Liu, Y. Self-assembly of Poly (Styrene-methyl Methacrylate-acrylic Acid) (P(St-MMA-AA)) Colloidal Microspheres on Wood Surface by Thermal-assisted Gravity Deposition. Wood Sci. Technol. 2021, 55, 403–417. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J. Investigation of Polystyrene-based Microspheres from Different Copolymers and Their Structural Color Coatings on Wood Surface. Coatings 2021, 11, 14. [Google Scholar] [CrossRef]
- Yan, X.; Qian, X.; Wu, Z. Self-repairing Technology of Microencapsulate and its Applications in Coatings. J. For. Eng. 2019, 4, 20–28. [Google Scholar]
- Siva, T.; Sathiyanarayanan, S. Self Healing Coatings Containing Dual Active Agent Loaded Urea Formaldehyde (UF) Microcapsules. Prog. Org. Coat. 2015, 82, 57–67. [Google Scholar] [CrossRef]
- Lang, S.; Zhou, Q. Synthesis and Characterization of Poly(urea-formaldehyde) Microcapsules Containing Linseed Oil for Self-healing Coating Development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Yang, H.-I.; Kim, D.-M.; Yu, H.-C.; Chung, C.-M. Microcapsule-Type Organogel-Based Self-Healing System Having Secondary Damage Preventing Capability. ACS Appl. Mater. Inter. 2016, 8, 11070–11075. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, Z. Fabrication of Coatings with Structural Color on A Wood Surface. Coatings 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Shao, Q.; Wu, M.; Zheng, B.; Guo, Z.; Yi, J.; Zhang, J.; Lin, J.; Wu, S.; Dong, M.; et al. 2-(3,4-Epoxy) Ethyltriethoxysilane-modified Waterborne Acrylic Resin: Preparation and Property Analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- Liu, M.; Mao, X.; Zhu, H.; Lin, A.; Wang, D. Water and Corrosion Resistance of Epoxy–acrylic–amine Waterborne Coatings: Effects of Resin Molecular Weight, Polar Group and Hydrophobic Segment. Corros. Sci. 2013, 75, 106–113. [Google Scholar] [CrossRef]
- Yan, X.; Tao, Y.; Qian, X. Preparation and Optimization of Waterborne Acrylic Core Microcapsules for Waterborne Wood Coatings and Comparison with Epoxy Resin Core. Polymers 2020, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qian, X.; Chang, Y. Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings. Coatings 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Wang, L. Preparation of Shellac Resin Microcapsules Coated with Urea Formaldehyde Resin and Properties of Waterborne Paint Films for Tilia amurensis Rupr. Membranes 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chang, Y.; Qian, X. Preparation and Self-Repairing Properties of Urea Formaldehyde-Coated Epoxy Resin Microcapsules. Int. J. Polym. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Gao, D.; Xu, W. Effect of Sanding Processes on the Surface Properties of Modified Poplar Coated by Primer Compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]












| Experimental Materials | Molecular Mass (g/moL) | CAS | Manufacturer |
|---|---|---|---|
| citric acid monohydrate | 210.14 | 5949-29-1 | Nanjing Chemical Reagent Co., Ltd., Nanjing, China |
| triethanolamine | 149.19 | 102-71-6 | Guangzhou Jiangshun Chemical Technology Co., Ltd., Guangzhou, China |
| 37.0% formaldehyde | 30.03 | 50-00-0 | Guangzhou Jiangshun Chemical Technology Co., Ltd., Guangzhou, China |
| urea | 60.06 | 57-13-6 | Nanjing Chemical Reagent Co., Ltd., Nanjing, China |
| sodium dodecyl benzene sulfonate (SDBS) | 348.48 | 25155-30-0 | Xi’an Tianmao Chemical Co., Ltd., Xi’an, China |
| ethyl acetate | 88.11 | 141-78-6 | Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China |
| anhydrous ethanol | 46.07 | 64-17-5 | Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China |
| Level | Mass Ratio of Core to Shell | Reaction Temperature (°C) | Standing Time (d) |
|---|---|---|---|
| 1 | 0.42:1 | 50 | 1 |
| 2 | 0.67:1 | 70 | 5 |
| Sample | 37.0% Formaldehyde (g) | Urea (g) | Waterborne Coatings (g) | Sodium Dodecyl Benzene Sulfonate (g) | Emulsifier (mL) |
|---|---|---|---|---|---|
| 1# | 27.00 | 20.00 | 12.50 | 0.97 | 97.00 |
| 2# | 27.00 | 20.00 | 12.50 | 0.97 | 97.00 |
| 3# | 27.00 | 20.00 | 20.00 | 1.56 | 156.00 |
| 4# | 27.00 | 20.00 | 20.00 | 1.56 | 156.00 |
| 5# | 27.00 | 20.00 | 12.50 | 0.97 | 97.00 |
| 6# | 27.00 | 20.00 | 15.00 | 1.17 | 117.00 |
| 7# | 27.00 | 20.00 | 17.50 | 1.37 | 137.00 |
| 8# | 27.00 | 20.00 | 20.00 | 1.56 | 156.00 |
| 9# | 27.00 | 20.00 | 22.50 | 1.76 | 176.00 |
| 10# | 27.00 | 20.00 | 25.00 | 1.95 | 195.00 |
| 11# | 27.00 | 20.00 | 27.50 | 2.15 | 215.00 |
| Sample | Mass Ratio of Core to Shell | Reaction Temperature (°C) | Standing Time (d) | Output (g) |
|---|---|---|---|---|
| 1# | 0.42:1 | 50 | 1 | 28.76 ± 0.95 |
| 2# | 0.42:1 | 70 | 5 | 29.34 ± 1.02 |
| 3# | 0.67:1 | 50 | 5 | 33.32 ± 0.84 |
| 4# | 0.67:1 | 70 | 1 | 32.19 ± 0.92 |
| Mean 1 | 29.05 ± 1.38 | 31.04 ± 0.97 | 30.47 ± 0.78 | |
| Mean 2 | 32.75 ± 0.85 | 30.76 ± 0.99 | 31.33 ± 0.83 | |
| R | 3.70 ± 0.10 | 0.27 ± 0.01 | 0.85 ± 0.01 |
| Factor | Sum of Squared Deviations | Degrees of Freedom | F Ratio 1 | F Critical Value 2 | Significance |
|---|---|---|---|---|---|
| Mass ratio of core to shell | 13.727 | 1 | 180.618 | 161.000 | * |
| Reaction temperature (°C) | 0.076 | 1 | 1.000 | 161.000 | |
| Standing time (d) | 0.731 | 1 | 9.618 | 161.000 | |
| Error | 0.08 | 1 |
| Sample | Mass Ratio of Core to Shell | Reaction Temperature (°C) | Standing Time (d) | Clad Ratio (%) |
|---|---|---|---|---|
| 1# | 0.42:1 | 50 | 1 | 33.0 ± 1.0 |
| 2# | 0.42:1 | 70 | 5 | 37.0 ± 1.0 |
| 3# | 0.67:1 | 50 | 5 | 43.0 ± 1.4 |
| 4# | 0.67:1 | 70 | 1 | 40.0 ± 0.8 |
| Mean 1 | 35.00 ± 1.01 | 38.00 ± 1.10 | 36.50 ± 0.90 | |
| Mean 2 | 41.50 ± 0.76 | 38.50 ± 1.00 | 40.00 ± 1.18 | |
| R | 6.50 ± 0.18 | 0.50 ± 0.02 | 3.50 ± 0.06 |
| Factor | Sum of Squared Deviations | Degrees of Freedom | F Ratio | F Critical Value | Significance |
|---|---|---|---|---|---|
| Mass ratio of core to shell | 42.25 | 1 | 160.00 | 161.00 | |
| Reaction temperature (°C) | 0.25 | 1 | 1.00 | 161.00 | |
| Standing time (d) | 12.25 | 1 | 49.00 | 161.00 | |
| Error | 0.25 | 1 |
| Sample | Mass Ratio of Core to Shell | Core Material Mass (g) | Output (g) |
|---|---|---|---|
| 5# | 0.42:1 | 12.5 | 28.93 ± 0.40 |
| 6# | 0.50:1 | 15.0 | 31.22 ± 1.19 |
| 7# | 0.58:1 | 17.5 | 34.35 ± 1.04 |
| 8# | 0.67:1 | 20.0 | 37.80 ± 0.88 |
| 9# | 0.75:1 | 22.5 | 35.57 ± 0.76 |
| 10# | 0.83:1 | 25.0 | 33.43 ± 0.69 |
| 11# | 0.92:1 | 27.5 | 33.47 ± 0.76 |
| Sample | Mass Ratio of Core to Shell | Core Material Mass (g) | Clad Ratio (%) |
|---|---|---|---|
| 5# | 0.42:1 | 12.5 | 37.0 ± 1.2 |
| 6# | 0.50:1 | 15.0 | 46.0 ± 1.4 |
| 7# | 0.58:1 | 17.5 | 45.0 ± 1.3 |
| 8# | 0.67:1 | 20.0 | 49.0 ± 1.8 |
| 9# | 0.75:1 | 22.5 | 42.0 ± 1.2 |
| 10# | 0.83:1 | 25.0 | 41.0 ± 1.3 |
| 11# | 0.92:1 | 27.5 | 32.0 ± 0.6 |
| Core Material | Optimum Mass Ratio of Core to Shell | Microcapsules Concentration (%) | Color Difference | Gloss (%) | Hardness | Adhesion (Grade) | Impact Resistance (kg·cm) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|---|
| − | − | 0 | 0.60 ± 0.01 | 29.4 ± 0.8 | HB ± 0 | 0 ± 0 | 6.0 ± 0.1 | 9.9 ± 0.2 |
| Waterborne coating | 0.67 | 10.0 | 2.00 ± 0.08 | 11.9 ± 0.3 | 3H ± 0 | 0 ± 0 | 13.0 ± 0.4 | 16.2 ± 0.5 |
| Waterborne acrylic acid | 0.58:1 | 10.0 | 1.80 ± 0.03 | 5.1 ± 0.1 | 2H ± 0 | 1 ± 0 | 15.0 ± 0.3 | 16.7 ± 0.5 |
| Epoxy resin | 0.83:1 | 10.0 | 3.50 ± 0.03 | 5.0 ± 0.1 | 5H ± 0 | 3 ± 0 | 20.0 ± 0.3 | 35.0 ± 1.0 |
| Shellac 1 | 0.75:1 | 10.0 | 1.50 ± 0.04 | 7.8 ± 0.2 | B ± 0 | 1 ± 0 | 9.0 ± 0.2 | 20.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Tao, Y.; Qian, X. Effect of Microcapsules with Waterborne Coating as Core Material on Properties of Coating for Tilia Europaea and Comparison with Other Microcapsules. Polymers 2021, 13, 3167. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183167
Yan X, Tao Y, Qian X. Effect of Microcapsules with Waterborne Coating as Core Material on Properties of Coating for Tilia Europaea and Comparison with Other Microcapsules. Polymers. 2021; 13(18):3167. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183167
Chicago/Turabian StyleYan, Xiaoxing, Yu Tao, and Xingyu Qian. 2021. "Effect of Microcapsules with Waterborne Coating as Core Material on Properties of Coating for Tilia Europaea and Comparison with Other Microcapsules" Polymers 13, no. 18: 3167. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183167