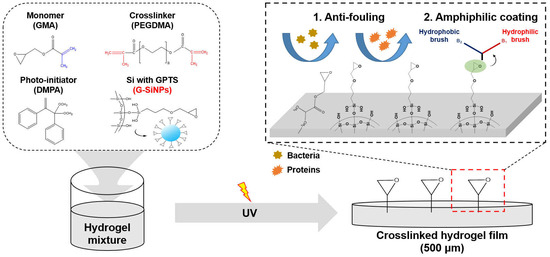
Effect of Silica Nanoparticles Blocked with Epoxy Groups on the Crosslinking and Surface Properties of PEG Hydrogel Films
Abstract
:1. Introduction
2. Experimental Process
2.1. Preparation of Si-NPs Blocked with GPTS
2.2. Formulation of UV Curable Hydrogel Mixtures
2.3. Chemo-Rheological Properties of Hydrogel Mixtures during UV Irradiation
2.4. Surface Analysis of Crosslinked Hydrogel Films
2.5. Surface Mechanical Properties of Crosslinked Hydrogel Films
3. Results and Discussion
3.1. Structural Features of G-SiNPs
3.2. Real-Time Rheological Properties of Hydrogel Mixtures during UV Irradiation
3.3. Distribution of Epoxy Units on Crosslinked Film Surface
3.4. Surface Mechanical Properties of Crosslinked Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Puperi, D.S.; Yonezawa, A.L.; Wu, Y.; Tseng, H.; Grande-Allen, K.J. Integrating valve-inspired design features into poly (ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015, 14, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, L.; Wan, X.; Jiang, X.; Yuan, J. Biocompatible and photocrosslinkable poly (ethylene glycol)/keratin biocomposite hydrogels. J. Biomater. Sci.-Polym. Ed. 2021, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.; Cao, Z.; Devine, D.M.; Major, I. Preparation of biodegradable polyethylene glycol dimethacrylate hydrogels via thiol-ene chemistry. Polymers 2019, 11, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Lee, B.H.; Irvine, S.A.; Wong, Y.S.; Bianco Peled, H.; Venkatraman, S. Inclusion of cross-linked elastin in Gelatin/PEG hydrogels favourably influences fibroblast phenotype. Polymers 2020, 12, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.W.; Noh, S.M.; Kim, B.; Jung, H.W. Gelation and crosslinking characteristics of photopolymerized poly (ethylene glycol) hydrogels. J. Appl. Polym. Sci. 2015, 132, 41939. [Google Scholar] [CrossRef]
- Jung, K.I.; Lee, D.G.; Bong, K.W.; Noh, S.M.; Um, M.S.; Choi, W.J.; Jung, H.W. Effects of solvents on rheological and crosslinking properties of photo-polymerized poly (ethylene glycol) hydrogels. Korean J. Chem. Eng. 2017, 34, 1517–1523. [Google Scholar] [CrossRef]
- Sung, J.; Lee, D.G.; Lee, S.; Park, J.; Jung, H.W. Crosslinking dynamics and gelation characteristics of photo-and thermally polymerized poly (ethylene glycol) hydrogels. Materials 2020, 13, 3277. [Google Scholar] [CrossRef]
- Pfister, P.M.; Wendlandt, M.; Neuenschwander, P.; Suter, U.W. Surface-textured PEG-based hydrogels with adjustable elasticity: Synthesis and characterization. Biomaterials 2007, 28, 567–575. [Google Scholar] [CrossRef]
- Gudipati, C.S.; Greenlief, C.M.; Johnson, J.A.; Prayongpan, P.; Wooley, K.L. Hyperbranched fluoropolymer and linear poly (ethylene glycol) based amphiphilic crosslinked networks as efficient antifouling coatings: An insight into the surface compositions, topographies, and morphologies. J. Polym. Sci. Pol. Chem. 2004, 42, 6193–6208. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that determine the protein resistance of oligoether self-assembled monolayers− internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, S.; Sasaki, M.; Saito, K.; Sugita, K.; Sugo, T. Amino acid addition to epoxy-group-containing polymer chain grafted 319 onto a porous membrane. J. Membr. Sci. 1996, 109, 87–92. [Google Scholar] [CrossRef]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; López-Fabal, F.; Fernández-García, M. Hemolytic and antimicrobial activities of a series of cationic amphiphilic copolymers comprised of same centered comonomers with thiazole moieties and polyethylene glycol derivatives. Polymers 2020, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Yoon, S.; Jung, K.I.; Lee, D.G.; Bang, J.; Jung, H.W. Crosslinking behaviors and mechanical properties of curable PDMS and PEG films with various contents of glycidyl methacrylate. J. Appl. Polym. Sci. 2019, 136, 47088. [Google Scholar] [CrossRef]
- Lee, D.G.; An, S.Y.; Um, M.S.; Choi, W.J.; Noh, S.M.; Jung, H.W.; Oh, J.K. Photo-induced thiol-ene crosslinked polymethacrylate networks reinforced with Al2O3 nanoparticles. Polymer 2016, 101, 119–126. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D-Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef] [Green Version]
- Allahverdi, A.; Ehsani, M.; Janpour, H.; Ahmadi, S. The effect of nanosilica on mechanical, thermal and morphological properties of epoxy coating. Prog. Org. Coat. 2012, 75, 543–548. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Chen, M.; Xue, Q.; Liu, W. Preparation and self-assembly of carboxylic acid-functionalized silica. J. Colloid Interface Sci. 2007, 311, 507–513. [Google Scholar] [CrossRef]
- Rosen, J.E.; Gu, F.X. Surface functionalization of silica nanoparticles with cysteine: A low-fouling zwitterionic surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef]
- Chu, L.; Daniels, M.W.; Francis, L.F. Use of (glycidoxypropyl) trimethoxysilane as a binder in colloidal silica coatings. Chem. Mat. 1997, 9, 2577–2582. [Google Scholar] [CrossRef]
- Chiou, B.S.; Raghavan, S.R.; Khan, S.A. Effect of colloidal fillers on the cross-linking of a UV-curable polymer: Gel point rheology and the winter− chambon criterion. Macromolecules 2001, 34, 4526–4533. [Google Scholar] [CrossRef] [Green Version]
- Wilson, O.R.; McDaniel, R.M.; Rivera, A.D.; Magenau, A.J. Alkylborane-initiated thiol-ene networks for the synthesis of thick and highly loaded nanocomposites. ACS Appl. Mater. Interfaces 2020, 12, 55262–55268. [Google Scholar] [CrossRef]
- Jung, K.I.; Hwang, S.O.; Kim, N.H.; Lee, D.G.; Lee, J.H.; Jung, H.W. Effect of methacryloxypropyl and phenyl functional groups on crosslinking and rheological and mechanical properties of ladder-like polysilsesquioxane hard coatings. Prog. Org. Coat. 2018, 124, 129–136. [Google Scholar] [CrossRef]
- Arizio, E.; Orsega, E.F.; Sommariva, G.; Falcone, R. Tin amalgam mirrors: Investigation by XRF, SEM-EDS, XRD and EPMA-WDS mapping. Appl. Phys. A-Mater. Sci. Process. 2013, 111, 733–745. [Google Scholar] [CrossRef]
- Fay, F.; Linossier, I.; Langlois, V.; Haras, D.; Vallee-Rehel, K. SEM and EDX analysis: Two powerful techniques for the study of antifouling paints. Prog. Org. Coat. 2005, 54, 216–223. [Google Scholar] [CrossRef]
- Xu, L.; Fu, J.H.; Schlup, J.R. In situ near-infrared spectroscopic investigation of the kinetics and mechanisms of reactions between phenyl glycidyl ether (PGE) and multifunctional aromatic amines. Ind. Eng. Chem. Res. 1996, 35, 963–972. [Google Scholar] [CrossRef]
- Caldona, E.B.; Wipf, D.O.; Smith, D.W., Jr. Characterization of a tetrafunctional epoxy-amine coating for corrosion protection of mild steel. Prog. Org. Coat. 2021, 151, 106045. [Google Scholar] [CrossRef]
- Boussu, K.; Van der Bruggen, B.; Volodin, A.; Snauwaert, J.; Van Haesendonck, C.; Vandecasteele, C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of AFM. J. Colloid Interface Sci. 2005, 286, 632–638. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Peppas, N.A. Polymerization kinetics and volume relaxation behavior of photopolymerized multifunctional monomers producing highly crosslinked networks. J. Polym. Sci. Pol. Chem. 1994, 32, 139–147. [Google Scholar] [CrossRef]
- Ebenstein, D.M.; Pruitt, L.A. Nanoindentation of biological materials. Nano Today 2006, 1, 26–33. [Google Scholar] [CrossRef]
- Consiglio, R.; Randall, N.X.; Bellaton, B.; Von Stebut, J. The nano-scratch tester (NST) as a new tool for assessing the strength of ultrathin hard coatings and the mar resistance of polymer films. Thin Solid Films. 1998, 332, 151–156. [Google Scholar] [CrossRef]
- Noh, S.M.; Lee, J.W.; Nam, J.H.; Park, J.M.; Jung, H.W. Analysis of scratch characteristics of automotive clearcoats containing silane modified blocked isocyanates via carwash and nano-scratch tests. Prog. Org. Coat. 2012, 74, 192–203. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Measuring, Mechanistic Aspects of Scratch/Mar Behavior of Paint Coatings by Nanoscratching; ASTM D 7187-15; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Yegorov, A.S.; Ivanov, V.S.; Antipov, A.V.; Wozniak, A.I.; Tcarkova, K.V. Chemical modification methods of nanoparticles of silicon carbide surface. Orient. J. Chem. 2015, 31, 1269–1275. [Google Scholar] [CrossRef]
- Afzal, A.; Siddiqi, H.M.; Saeed, S.; Ahmad, Z. The influence of epoxy functionalized silica nanoparticles on stress dispersion and crack resistance in epoxy–based hybrids. Mater. Express 2011, 1, 299–306. [Google Scholar] [CrossRef]
- Lin, P.; Ding, L.; Lin, C.W.; Gu, F. Nonfouling property of zwitterionic cysteine surface. Langmuir 2014, 30, 6497–6507. [Google Scholar] [CrossRef]








| Sample | PEGDMA (g) | GMA (g) | DMPA (g) | G-SiNPs (g) |
|---|---|---|---|---|
| PEG 0 | 0.90 | 0.10 | 0.01 | 0.00 |
| PEG 1 | 0.01 | |||
| PEG 2 | 0.02 | |||
| PEG 5 | 0.05 | |||
| PEG 7 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, N.; Jung, K.I.; Yoon, S.; Noh, S.M.; Bang, J.; Jung, H.W. Effect of Silica Nanoparticles Blocked with Epoxy Groups on the Crosslinking and Surface Properties of PEG Hydrogel Films. Polymers 2021, 13, 3296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193296
Park J, Kim N, Jung KI, Yoon S, Noh SM, Bang J, Jung HW. Effect of Silica Nanoparticles Blocked with Epoxy Groups on the Crosslinking and Surface Properties of PEG Hydrogel Films. Polymers. 2021; 13(19):3296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193296
Chicago/Turabian StylePark, Junyoung, Nahee Kim, Kevin Injoe Jung, Soomin Yoon, Seung Man Noh, Joona Bang, and Hyun Wook Jung. 2021. "Effect of Silica Nanoparticles Blocked with Epoxy Groups on the Crosslinking and Surface Properties of PEG Hydrogel Films" Polymers 13, no. 19: 3296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193296