Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes
Abstract
:1. Introduction
2. Materials and Methods
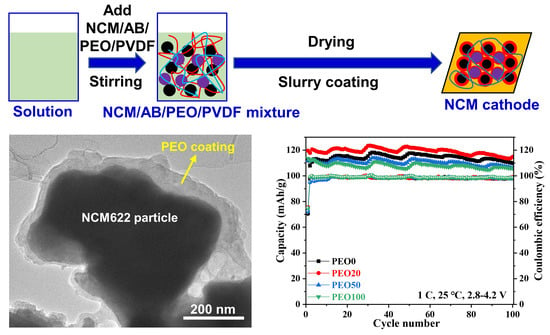
2.1. Preparation of PEO-Containing Electrodes
2.2. Preparation of PEO-Coated NCM622 Particle and PEO-Based Electrodes
2.3. Assembly of Lithium-Ion Batteries
2.4. Material Characterizations
3. Results and Discussion
3.1. Preparation and Properties of the PEO-Based Electrolytes and NCM622 Particles
3.2. Micromorphology of the PEO-Containing Cathodes
3.3. Electrochemical Properties of the PEO-Containing Batteries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Liu, N.; Chen, L.; Li, N.; Dong, J.; Lu, Y.; Tan, G.; Xu, M.; Cao, D.; Liu, Y.; et al. The nature of irreversible phase transformation propagation in nickel-rich layered cathode for lithium-ion batteries. J. Energy Chem. 2021, 62, 351–358. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Liu, Y.; Yao, N.; Li, J.; Liu, Y. Enhancement of the high-voltage electrochemical performance of an LiNi0.5Co0.2Mn0.3O2 cathode via WO3 coating. Appl. Surf. Sci. 2020, 508, 145259. [Google Scholar]
- Kim, U.-H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Extending the battery life using an Al-doped Li[Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium ion batteries. ACS Energy Lett. 2017, 2, 1848–1854. [Google Scholar]
- Wang, D.; Liu, M.; Wang, X.; Yu, R.; Wang, G.; Ren, Q.; Yang, X. Facile synthesis and performance of Na-doped porous lithium-rich cathodes for lithium ion batteries. RSC Adv. 2016, 6, 57310–57319. [Google Scholar] [CrossRef]
- Sivajee-Ganesh, K.; Purusottam-Reddy, B.; Hussain, O.M.; Mauger, A.; Julien, C.M. Influence of Ti and Zr dopants on the electrochemical performance of LiCoO2 film cathodes prepared by RF-magnetron sputtering. Mater. Sci. Eng. B 2016, 209, 30–36. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Wang, Z.; Guo, H.; Xu, Y.; Fan, Y.; Ru, J. Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim. Acta 2016, 188, 48–56. [Google Scholar] [CrossRef]
- Pang, S.; Wang, Y.; Chen, T.; Shen, X.; Xi, X.; Liao, D. The effect of AlF3 modification on the physicochemical and electrochemical properties of Li-rich layered oxide. Ceram. Int. 2016, 42, 5397–5402. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Xue, Z.-C.; Yang, L.; Wang, J.; Meng, F.; Li, Q.; Pan, H.; Zhang, J.-N.; Jiang, Z.; et al. An in situ formed surface coating layer enabling LiCoO2 with stable 4.6 V high-voltage cycle performances. Adv. Energy Mater. 2020, 10, 2001413. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, J.; Lu, J.; Li, Y.; Yan, P.; Zhang, Y. Realizing superior cycling stability of Ni-rich layered cathode by combination of grain boundary engineering and surface coating. Nano Energy 2019, 62, 30–37. [Google Scholar] [CrossRef]
- Yuan, A.; Tang, H.; Liu, L.; Ying, J.; Tan, L.; Tan, L.; Sun, R. High performance of phosphorus and fluorine co-doped LiNi0.8Co0.1Mn0.1O2 as a cathode material for lithium ion batteries. J. Alloy. Compd. 2020, 844, 156210. [Google Scholar] [CrossRef]
- Li, C.-D.; Xu, J.; Xia, J.-S.; Liu, W.; Xiong, X.; Zheng, Z.-A. Influences of FeF3 coating layer on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium-ion batteries. Solid State Ion. 2016, 292, 75–82. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Su, Z.; Hencz, L.; Chen, S.; Yan, C.; Zhang, S. A hydrophilic poly(methyl vinyl ether-alt-maleic acid) polymer as a green, universal, and dual-functional binder for high-performance silicon anode and sulfur cathode. J. Energy Chem. 2021, 62, 127–135. [Google Scholar] [CrossRef]
- Chu, H.; Lee, K.; Lim, S.; Kim, T.-H. Enhancing the performance of a silicon anode by using a new conjugated polymer binder prepared by direct arylation. Macromol. Res. 2018, 26, 738–743. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Liu, Y.-L. 2,2-dimethyl-1,3-dioxane-4,6-dione functionalized poly(ethylene oxide)-based polyurethanes as multi-functional binders for silicon anodes of lithium ion batteries. Electrochim. Acta 2021, 379, 138180. [Google Scholar] [CrossRef]
- Sun, S.; He, D.; Li, P.; Liu, Y.; Wan, Q.; Tan, Q.; Liu, Z.; An, F.; Gong, G.; Qu, X. Improved adhesion of cross-linked binder and SiO2-coating enhances structural and cyclic stability of silicon electrodes for lithium-ion batteries. J. Power Sources 2020, 454, 227907. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, W.; Wang, F.; Wang, J. Enhanced electrochemical properties of LiFePO4 (LFP) cathode using the carboxymethyl cellulose lithium (CMC-Li) as novel binder in lithium-ion battery. Carbohyd. Polym. 2014, 111, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.N.; Apraksin, R.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Electrochemical impedance spectroscopy characterization of LiFePO4 cathode material with carboxymethylcellulose and poly-3,4-ethylendioxythiophene/polystyrene sulfonate. Electrochim. Acta 2017, 227, 357–366. [Google Scholar] [CrossRef]
- Ma, T.; Yu, X.; Cheng, X.; Li, H.; Zhu, W.; Qiu, X. Confined solid electrolyte interphase growth space with solid polymer electrolyte in hollow structured silicon anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 13247–13254. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lyu, J.; Mo, J.; Yan, H.; Xu, L.; Peng, P.; Li, J.; Jiang, B.; Chu, L.; Li, M. Comprehensively-upgraded polymer electrolytes by multifunctional aramid nanofibers for stable all-solid-state Li-ion batteries. Nano Energy 2020, 69, 104398. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liang, Y.; Han, Z.; Liu, S.; Chu, W.; Yu, H. Interphase engineering by electrolyte additives for lithium-rich layered oxides: Advances and perspectives. ACS Energy Lett. 2021, 6, 2552–2564. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R.; Zhu, X.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R. Facile fabrication of flexible Si-based nanocomposite films as high-rate anodes by layer-by-layer self-assembly. Appl. Surf. Sci. 2019, 476, 501–512. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Zhang, D.; Sun, M.; Liu, L.; Hu, W.; Jiang, B.; Chu, L.; Li, M. Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes. Polymers 2021, 13, 3992. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13223992
Mo J, Zhang D, Sun M, Liu L, Hu W, Jiang B, Chu L, Li M. Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes. Polymers. 2021; 13(22):3992. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13223992
Chicago/Turabian StyleMo, Jinshan, Dongmei Zhang, Mingzhe Sun, Lehao Liu, Weihao Hu, Bing Jiang, Lihua Chu, and Meicheng Li. 2021. "Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes" Polymers 13, no. 22: 3992. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13223992