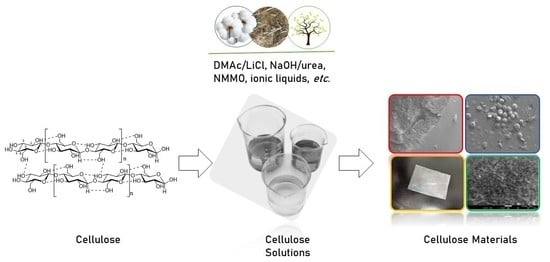
Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications
Abstract
:1. Introduction
1.1. The Sustainability Aspects of Cellulosic Materials
1.2. Limitations in Uses of Cellulose
2. Structural Characteristics of Cellulose Important for Dissolution
3. Important Factors in Cellulose Dissolution
3.1. Molecular Weight
3.2. Crystallinity
3.3. Hydrophobic Interactions
4. Cellulose Solvents
4.1. Aqueous Sodium Hydroxide (H2O-NaOH)-Based Solvent Systems
Strategies for Improvement of Cellulose Dissolution in NaOH-Based Solvents
4.2. N,N-Dimethylacetamide (DMAc)/Lithium Chloride (LiCl) System
Strategies for Improvement of Cellulose Dissolution in DMAc/LiCl Solvent System
| Cellulose Source | Cellulose DP | Pretreatment | Dissolution Conditions | Ref. | ||
|---|---|---|---|---|---|---|
| Cellulose Concentration (wt.%) | Temperature (°C) | Time (h) | ||||
| Wood pulp | ~800 | Solvent exchange with DMAc | 0.4 | 40 | 24 | [124] |
| Cotton | ~5350 | Freeze-drying | 3.0–5.0 | 80 | 74.5 | [105] |
| Cotton | ~2850 | Solvent exchange with methanol | 6.0–15.0 | RT | 24–48 | [97] |
| Cotton | 4580 | High-temperature dissolution | 2.2 | 150 | 2.5 | [125] |
| Cotton | ~11,300 | Heat activation | 1.2 | 150 | 48 | [120] |
| Cotton | ~1100 | Heat activation | 1.0 | 100 | 50 | [122] |
| Cotton | ~21,000 | Heat activation | 1.5 | 150 | 24–48 | [121] |
| Cotton | ~10,900 | High-temperature dissolution | 1.0 | 150 | 52 | [126] |
| MCC | ND | Freeze-drying | 5.0 | 80 | 24 | [8] |
4.3. N-Methylmorpholine-N-Oxide (NMMO)
Strategies for Improvement of Cellulose Dissolution in NMMO
4.4. Ionic Liquids (ILs)
Strategies for Improvement of Dissolution of Cellulose in IL-Based Systems
5. Cellulose Regeneration and Shaping
5.1. Cellulose Hydrogels
5.2. Aerocelluloses
5.3. Cellulose Membranes
5.4. Cellulose Fibrous Materials
6. Potential Impacts: Application of Cellulose Materials
6.1. Biomedical Applications
6.2. Sorption Applications
6.3. Energy Applications
6.4. Application in Thermal Insulations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Qi, H. Novel Functional Materials Based on Cellulose (SpringerBriefs in Applied Sciences and Technology), 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief Overview on Cellulose Dissolution/Regeneration Interactions and Mechanisms. Adv. Colloid Interface Sci. 2014, 222, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose Solvent-Based Pretreatment for Enhanced Second-Generation Biofuel Production: A Review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Rumi, S.S.; Liyanage, S.; Abidi, N. Conversion of Low-Quality Cotton to Bioplastics. Cellulose 2021, 28, 2021–2038. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Moussa, H.; Abidi, N. Preparation and Characterization of Transparent Cellulose Films Using an Improved Cellulose Dissolution Process. J. Appl. Polym. Sci. 2017, 134, 1–12. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from Synthetic Textiles as a Major Source of Microplastics in the Environment: A Review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.S.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as Vectors for Environmental Contaminants: Exploring Sorption, Desorption, and Transfer to Biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.M.A.; Abdel-Raouf, M.E. Applications of Guar Gum and Its Derivatives in Petroleum Industry: A Review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Tenenbaum, D.J. Food vs. Fuel: Diversion of Crops Could Cause More Hunger. Environ. Health Perspect. 2008, 116, A254–A257. [Google Scholar] [CrossRef] [Green Version]
- Malladi, R.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agriculture and Industrial Waste in the Field of Nano Cellulose and Its Recent Industrial Developments: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 135, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Liebert, T. Cellulose Solvents—Remarkable History, Bright Future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T.F., Heinze, T.J., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Y.; Shi, C.; Ye, Y.; Wang, P.; Zeng, X.; Wu, T. Preparation and Characterization of Cellulose Regenerated from Phosphoric Acid. J. Agric. Food Chem. 2013, 61, 12405–12414. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- French, A.D. Glucose, Not Cellobiose, Is the Repeating Unit of Cellulose and Why That Is Important. Cellulose 2017, 24, 4605–4609. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into Hydrogen Bonding and Stacking Interactions in Cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Johnson, G.P.; French, A.D.; Forsyth, V.T.; Langan, P. Neutron Crystallography, Molecular Dynamics, and Quantum Mechanics Studies of the Nature of Hydrogen Bonding in Cellulose Iβ. Biomacromolecules 2008, 9, 3133–3140. [Google Scholar] [CrossRef]
- Lu, B.; Xu, A.; Wang, J. Cation Does Matter: How Cationic Structure Affects the Dissolution of Cellulose in Ionic Liquids. Green Chem. 2014, 16, 1326–1335. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Ding, S.Y.; Himmel, M.E. The Maize Primary Cell Wall Microfibril: A New Model Derived from Direct Visualization. J. Agric. Food Chem. 2006, 54, 597–606. [Google Scholar] [CrossRef]
- Ding, S.Y.; Zhao, S.; Zeng, Y. Size, Shape, and Arrangement of Native Cellulose Fibrils in Maize Cell Walls. Cellulose 2014, 21, 863–871. [Google Scholar] [CrossRef]
- Fernandes, A.N.; Thomas, L.H.; Altaner, C.M.; Callow, P.; Forsyth, V.T.; Apperley, D.C.; Kennedy, C.J.; Jarvis, M.C. Nanostructure of Cellulose Microfibrils in Spruce Wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195–E1203. [Google Scholar] [CrossRef] [Green Version]
- Newman, R.H.; Hill, S.J.; Harris, P.J. Wide-Angle x-Ray Scattering and Solid-State Nuclear Magnetic Resonance Data Combined to Test Models for Cellulose Microfibrils in Mung Bean Cell Walls. Plant Physiol. 2013, 163, 1558–1567. [Google Scholar] [CrossRef] [Green Version]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T. Fungal Cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep Eutectic Solvents (DESs) for Cellulose Dissolution: A Mini-Review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Lindman, B.; Karlstromm, G.; Stigsson, L. On the Mechanism of Dissolution of Cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Competing Forces during Cellulose Dissolution: From Solvents to Mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Kauffman, G.B. Rayon: The First Semi-Synthetic Fiber Product. J. Chem. Educ. 1993, 70, 887–893. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of Cellulose Non-Derivatizing Solvent Interactions with Emphasis on Activity in Inorganic Molten Salt Hydrates. ACS Sustainable Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Liu, S.; Liu, Y.; Xu, X.; Chen, X.; Chu, B.; Guo, X.; Xu, J.; Cheng, H.; et al. Dynamic Self-Assembly Induced Rapid Dissolution of Cellulose at Low Temperatures. Macromolecules 2008, 41, 9345–9351. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-Methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Abidi, N. Mild Condition Dissolution of High Molecular Weight Cotton Cellulose in 1-Butyl-3-Methylimidazolium Acetate/N,N-Dimethylacetamide Solvent System. J. Appl. Polym. Sci. 2018, 135, 45928–45935. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent Effects on Cellulose Dissolution in Imidazolium-Based Ionic Liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Holmberg, K.; Jonsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution; John Wiley & Sons, Ltd.: Lund, Sweden, 2002. [Google Scholar]
- Olsson, C.; Westman, G. Direct Dissolution of Cellulose: Background, Means and Applications. In Cellulose—Fundamental Aspects; InTech Open: London, UK, 2013; pp. 143–178. [Google Scholar] [CrossRef] [Green Version]
- Shrotri, A.; Lambert, L.K.; Tanksale, A.; Beltramini, J. Mechanical Depolymerisation of Acidulated Cellulose: Understanding the Solubility of High Molecular Weight Oligomers. Green Chem. 2013, 15, 2761–2768. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B. The Subtleties of Dissolution and Regeneration of Cellulose: Breaking and Making Hydrogen Bonds. BioRes 2015, 10, 3811–3814. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–Water Based Solvents: A Review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Yang, Q.; Kuga, S.; Matsumoto, Y. Dissolution of Wood Pulp in Aqueous NaOH/Urea Solution via Dilute Acid Pretreatment. J. Agric. Food Chem. 2015, 63, 6113–6119. [Google Scholar] [CrossRef]
- Trygg, J.; Fardim, P. Enhancement of Cellulose Dissolution in Water-Based Solvent via Ethanol-Hydrochloric Acid Pretreatment. Cellulose 2011, 18, 987–994. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Tian, Z.; Zheng, X.; Chen, L. Controlled Production of Spruce Cellulose Gels Using an Environmentally "Green" System. Cellulose 2014, 21, 1667–1678. [Google Scholar] [CrossRef]
- Singh, P.; Duarte, H.; Alves, L.; Antunes, F.; Moigne, N.L.; Dormanns, J.; Duchemin, B.; Staiger, M.P.; Medronho, B. From Cellulose Dissolution and Regeneration to Added Value Applications—Synergism Between Molecular Understanding and Material Development. In Cellulose—Fundamental Aspects and Current Trends; Intech Open: London, UK, 2015; pp. 1–44. [Google Scholar]
- Acharya, S.; Hu, Y.; Abidi, N. Cellulose Dissolution in Ionic Liquid under Mild Conditions: Effect of Hydrolysis and Temperature. Fibers 2021, 9, 5. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lee, C.K. Enhancement of Enzymatic Saccharification of Cellulose by Cellulose Dissolution Pretreatments. Carbohydr. Polym. 2009, 77, 41–46. [Google Scholar] [CrossRef]
- Parviainen, H.; Parviainen, A.; Virtanen, T.; Kilpeläinen, I.; Ahvenainen, P.; Serimaa, R.; Grönqvist, S.; Maloney, T.; Maunu, S.L. Dissolution Enthalpies of Cellulose in Ionic Liquids. Carbohydr. Polym. 2014, 113, 67–76. [Google Scholar] [CrossRef]
- Ghasemi, M.; Tsianou, M.; Alexandridis, P. Assessment of Solvents for Cellulose Dissolution. Bioresour. Technol. 2017, 228, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.A.; Morgado, D.L.; Gessner, F.; Frollini, E.; El Seoudb, O.A. A Physical Organic Chemistry Approach to Dissolution of Cellulose: Effects of Cellulose Mercerization on Its Properties and on the Kinetics of Its Decrystallization. Arkivoc 2011, 7, 416–425. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.A.; Assaf, J.M.; El Seoud, O.A.; Frollini, E. Influence of the Supramolecular Structure and Physicochemical Properties of Cellulose on Its Dissolution in a Lithium Chloride/N,N-Dimethylacetamide Solvent System. Biomacromolecules 2005, 6, 2638–2647. [Google Scholar] [CrossRef]
- Buschle-Diller, G.; Zeronian, S.H. Enhancing the Reactivity and Strength of Cotton Fibers. J. Appl. Polym. Sci. 1992, 45, 967–979. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Effects of Temperature and Molecular Weight on Dissolution of Cellulose in NaOH/Urea Aqueous Solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of Solvent Exchange on the Solid Structure and Dissolution Behavior of Cellulose. Biomacromolecules 2003, 4, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Alexandridis, P.; Tsianou, M. Dissolution of Cellulosic Fibers: Impact of Crystallinity and Fiber Diameter. Biomacromolecules 2018, 19, 640–651. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation Studies of the Insolubility of Cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing Cellulose (In)Solubility: Reviewing Basic Physicochemical Aspects and Role of Hydrophobic Interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. General Considerations on Structure and Reactivity of Cellulose. In Comprehensive Cellulose Chemistry: Fundamentals and Analytical Methods, Volume 1; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998; p. 331. [Google Scholar]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A Critical Review of All-Cellulose Composites. J. Mater. Sci. 2011, 47, 1171–1186. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Grelier, S.S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability is not Enough. ACS Sustainable Chem. Eng. 2019, 7, 1826–1840. [Google Scholar] [CrossRef] [Green Version]
- Battista, O.A. Hydrolysis and Crystallization of Cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. Structure of Aqueous Solutions of Microcrystalline Cellulose/Sodium Hydroxide below 0 °C and the Limit of Cellulose Dissolution. Biomacromolecules 2007, 8, 2282–2287. [Google Scholar] [CrossRef]
- Le Moigne, N.; Navard, P. Dissolution Mechanisms of Wood Cellulose Fibres in NaOH–Water. Cellulose 2009, 17, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Egal, M.; Budtova, T.; Navard, P. The Dissolution of Microcrystalline Cellulose in Sodium Hydroxide-Urea Aqueous Solutions. Cellulose 2008, 15, 361–370. [Google Scholar] [CrossRef]
- Kamide, K.; Okajima, K.; Kowsaka, K. Dissolution of Natural Cellulose into Aqueous Alkali Solution: Role of Super-Molecular Structure of Cellulose. Polym. J. 1992, 24, 71–86. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Li, F.; Yu, J. Swelling and Dissolution of Cellulose in NaOH Aqueous Solvent System. Cellul. Chem. Technol. 2013, 47, 671–679. [Google Scholar]
- Kamida, K.; Kunihiko, K.; Matsui, T.; Kowsaka, K. Study on the Solubility of Cellulose in Aqueous Alkali Solution by Deuteration IR and 13C NMR. Polym. J. 1984, 16, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A.; Atalla, R.H. Dissolution of Cellulose in Aqueous NaOH Solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Davidson, G.F. 12—The Dissolution of Chemically Modified Cotton Cellulose in Alkaline Solutions. Part I—in Solutions of Sodium Hydroxide, Particularly at Temperatures Below the Normal. J. Text. Inst. Trans. 1934, 25, T174–T196. [Google Scholar] [CrossRef]
- Fink, H.P.; Walenta, E.; Kunze, J.; Mann, G. Wide Angle X-Ray and Solid State 13C-NMR Studies of Cellulose Alkalization. In Cellulose and Cellulose Derivatives. Physico-Chemical Aspects and Industrial Applications; Woodhead Publishing Ltd.: Cambridge, UK, 1995; pp. 523–528. [Google Scholar]
- Isogai, A. NMR Analysis of Cellulose Dissolved in Aqueous NaOH Solutions. Cellulose 1997, 4, 99–107. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Cai, P.; Zhang, L.; Hu, K.; Cheng, G. NMR Spectroscopic Studies on the Mechanism of Cellulose Dissolution in Alkali Solutions. Cellulose 2013, 20, 613–621. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, L. Unique Gelation Behavior of Cellulose in NaOH/Urea Aqueous Solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef]
- Lu, A.; Liu, Y.; Zhang, L.; Potthast, A. Investigation on Metastable Solution of Cellulose Dissolved in NaOH/Urea Aqueous System at Low Temperature. J. Phys. Chem. B 2011, 115, 12801–12808. [Google Scholar] [CrossRef]
- Lue, A.; Liu, Y.; Zhang, L.; Potthas, A. Light Scattering Study on the Dynamic Behaviour of Cellulose Inclusion Complex in LiOH/Urea Aqueous Solution. Polymer 2011, 52, 3857–3864. [Google Scholar] [CrossRef]
- Wawro, D.; Steplewski, W.; Bodek, A. Manufacture of Cellulose Fibres from Alkaline Solutions of Hydrothermally—Treated Cellulose Pulp. Fibres Text. East. Eur. 2009, 74, 18–22. [Google Scholar]
- Yamashiki, T.; Matsui, T.; Saitoh, M.; Okajima, K.; Kamide, K.; Sawada, T. Characterisation of Cellulose Treated by the Steam Explosion Method. Part 1: Influence of Cellulose Resources on Changes in Morphology, Degree of Polymerisation, Solubility and Solid Structure. Br. Polym. J. 1990, 22, 73–83. [Google Scholar] [CrossRef]
- Yamashiki, T.; Matsui, T.; Saitoh, M.; Okajima, K.; Kamide, K.; Sawada, T. Characterisation of Cellulose Treated by the Steam Explosion Method. Part 2: Effect of Treatment Conditions on Changes in Morphology, Degree of Polymerisation, Solubility in Aqueous Sodium Hydroxide and Supermolecular Structure of Soft Wood Pulp during St. Br. Polym. J. 1990, 22, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Deng, Y. Effect of Enzymatic Treatment on Cotton Fiber Dissolution in NaOH/Urea Solution at Cold Temperature. Carbohydr. Polym. 2008, 72, 178–184. [Google Scholar] [CrossRef]
- Le Moigne, N.; Jardeby, K.; Navard, P. Structural Changes and Alkaline Solubility of Wood Cellulose Fibers after Enzymatic Peeling Treatment. Carbohydr. Polym. 2010, 79, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Liu, Y.; Xu, H.; Yang, Q.; Xiong, C.; Kuga, S.; Matsumoto, Y. Facile Dissolution of Wood Pulp in Aqueous NaOH/Urea Solution by Ball Milling Pretreatment. Ind. Crop. Prod. 2018, 118, 48–52. [Google Scholar] [CrossRef]
- Qi, H.; Yang, Q.; Zhang, L.; Liebert, T.; Heinze, T. The Dissolution of Cellulose in NaOH-Based Aqueous System by Two-Step Process. Cellulose 2011, 18, 237–245. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Zhou, J.; Li, H.; Chen, H.; Jin, H. Novel Fibers Prepared from Cellulose in NaOH/Urea Aqueous Solution. Macromol. Rapid Commun. 2004, 25, 1558–1562. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, H.; Lue, A.; Hu, K.; Cheng, G.; Zhang, L. Role of Sodium Zincate on Cellulose Dissolution in NaOH/Urea Aqueous Solution at Low Temperature. Carbohydr. Polym. 2011, 83, 1185–1191. [Google Scholar] [CrossRef]
- Kihlman, M.; Wallberg, O.; Stigsson, L.; Germgård, U. Dissolution of Dissolving Pulp in Alkaline Solvents after Steam Explosion Pretreatments. Holzforschung 2011, 65, 613–617. [Google Scholar] [CrossRef]
- Ruan, D.; Zhang, L.; Zhou, J.; Jin, H.; Chen, H. Structure and Properties of Novel Fibers Spun from Cellulose in NaOH/Thiourea Aqueous Solution. Macromol. Biosci. 2004, 4, 1105–1112. [Google Scholar] [CrossRef]
- Yan, L.; Gao, Z. Dissolving of Cellulose in PEG/NaOH Aqueous Solution. Cellulose 2008, 15, 789–796. [Google Scholar] [CrossRef]
- Swensson, B.; Larsson, A.; Hasani, M. Dissolution of Cellulose Using a Combination of Hydroxide Bases in Aqueous Solution. Cellulose 2020, 27, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Dawsey, T.R.; McCormick, C.L. The Lithium Chloride/Dimethylacetamide Solvent for Cellulose: A Literature Review. J. Macromol. Sci. Part C 1990, 30, 405–440. [Google Scholar] [CrossRef]
- Spange, S.; Reuter, A.; Vilsmeier, E.; Heinze, T.; Keutel, D.; Linert, W. Determination of Empirical Polarity Parameters of the Cellulose Solvent N,N-Dimethylacetamide/LiCl by Means of the Solvatochromic Technique. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1945–1955. [Google Scholar] [CrossRef]
- McCormick, C.L.; Callais, P.A. Derivatization of Cellulose in Lithium Chloride and N,N-Dimethylacetamide Solutions. Polymer 1987, 28, 2317–2323. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Callais, P.A.; Hutchinson, B.H. Solution Studies of Cellulose in Lithium Chloride and N,N-Dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Patkar, S.N.; Panzade, P.D.; Silva, A.A.; Laver, M.L.; Marcus, B.F.; Chistopher, A.H.; Art, J.R.; Moore, J.C.; Leaca, A.A.; O’Shea, P.; et al. Fast and Efficient Method for Molecular Weight Analysis of Cellulose Pulp, in-Process and Finished Product. Anal. Methods 2016, 8, 3210–3215. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Marson, G.A.; Ciacco, G.T.; Frollini, E. An Efficient, One-Pot Acylation of Cellulose under Homogeneous Reaction Conditions. Macromol. Chem. Phys. 2000, 201, 882–889. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A Critical Review of Manufacturing Processes Used in Regenerated Cellulosic Fibres: Viscose, Cellulose Acetate, Cuprammonium, LiCl/DMAc, Ionic Liquids, and NMMO Based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Chrapava, S.; Touraud, D.; Rosenau, T.; Potthast, A.; Kunz, W. The Investigation of the Influence of Water and Temperature on the LiCl/DMAc/Cellulose System. Phys. Chem. Chem. Phys. 2003, 5, 1842–1847. [Google Scholar] [CrossRef]
- Röder, T.; Potthast, A.; Rosenau, T.; Kosmsa, P.; Baldinger, T.; Morgenstern, B.; Glatter, O. The Effect of Water on Cellulose Solutions in DMAc/LiCl. Macromol. Symp. 2002, 159, 151–159. [Google Scholar] [CrossRef]
- Sjöholm, E.; Gustafsson, K.; Eriksson, B.; Brown, W.; Colmsjö, A. Aggregation of Cellulose in Lithium Chloride/N,N-Dimethylacetamide. Carbohydr. Polym. 2000, 41, 153–161. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sixta, H.; Kosma, P. Degradation of Cellulosic Materials by Heating in DMAc/LiCl. Tetrahedron Lett. 2002, 43, 7757–7759. [Google Scholar] [CrossRef]
- Hu, Y.; Acharya, S.; Abidi, N. Cellulose Porosity Improves Its Dissolution by Facilitating Solvent Diffusion. Int. J. Biol. Macromol. 2019, 123, 1289–1296. [Google Scholar] [CrossRef]
- Potthast, A.; Radosta, S.; Saake, B.; Lebioda, S.; Heinze, T.; Henniges, U.; Isogai, A.; Koschella, A.; Kosma, P.; Rosenau, T.; et al. Comparison Testing of Methods for Gel Permeation Chromatography of Cellulose: Coming Closer to a Standard Protocol. Cellulose 2015, 22, 1591–1613. [Google Scholar] [CrossRef]
- Ono, Y.; Ishida, T.; Soeta, H.; Saito, T.; Isogai, A. Reliable dn/dc Values of Cellulose, Chitin, and Cellulose Triacetate Dissolved in LiCl/N,N-Dimethylacetamide for Molecular Mass Analysis. Biomacromolecules 2016, 17, 192–199. [Google Scholar] [CrossRef]
- Henniges, U.; Kostic, M.; Borgards, A.; Rosenau, T.; Potthastt, A. Dissolution Behavior of Different Celluloses. Biomacromolecules 2011, 12, 871–879. [Google Scholar] [CrossRef]
- Hasani, M.; Henniges, U.; Idström, A.; Nordstierna, L.; Westman, G.; Rosenau, T.; Potthast, A. Nano-Cellulosic Materials: The Impact of Water on Their Dissolution in DMAc/LiCl. Carbohydr. Polym. 2013, 98, 1565–1572. [Google Scholar] [CrossRef]
- Aulin, C.; Ahok, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Österberg, M.; Wågberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures—Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Gindl, W.; Emsenhuber, G.; Maier, G.; Keckes, J. Cellulose in Never-Dried Gel Oriented by an AC Electric Field. Biomacromolecules 2009, 10, 1315–1318. [Google Scholar] [CrossRef]
- Hassan, M.L.; Moorefield, C.N.; Kotta, K.; Newkome, G.R. Regioselective Combinatorial-Type Synthesis, Characterization, and Physical Properties of Dendronized Cellulose. Polymer 2005, 46, 8947–8955. [Google Scholar] [CrossRef]
- Ramos, L.A.; Morgado, D.L.; El Seoud, O.A.; da Silva, V.C.; Frollini, E. Acetylation of Cellulose in LiCl—N,N-Dimethylacetamide: First Report on the Correlation between the Reaction Efficiency and the Aggregation Number of Dissolved Cellulose. Cellulose 2011, 18, 385–392. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N,N-Dimethylacetamide/Lithium Chloride: Revisiting through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef]
- Strlič, M.; Kolar, J. Size Exclusion Chromatography of Cellulose in LiCl/N,N-Dimethylacetamide. J. Biochem. Biophys. Methods 2003, 56, 265–279. [Google Scholar] [CrossRef]
- Röder, T.; Morgenstern, B.; Schelosky, N.; Glatter, O. Solutions of Cellulose in N,N-Dimethylacetamide/Lithium Chloride Studied by Light Scattering Methods. Polymer 2001, 42, 6765–6773. [Google Scholar] [CrossRef]
- Dupont, A.L. Cellulose in Lithium Chloride/N,N-Dimethylacetamide, Optimisation of a Dissolution Method Using Paper Substrates and Stability of the Solutions. Polymer 2003, 44, 4117–4126. [Google Scholar] [CrossRef]
- Hu, Y.; Thalangamaarachchige, V.D.; Acharya, S.; Abidi, N. Role of Low-Concentration Acetic Acid in Promoting Cellulose Dissolution. Cellulose 2018, 25, 4389–4405. [Google Scholar] [CrossRef]
- Raus, V.; Šturcová, A.; Dybal, J.; Šlouf, M.; Vacková, T.; Šálek, P.; Kobera, L.; Vlček, P. Activation of Cellulose by 1,4-Dioxane for Dissolution in N,N-Dimethylacetamide/LiCl. Cellulose 2012, 19, 1893–1906. [Google Scholar] [CrossRef]
- Timpa, J.D. Application of Universal Calibration in Gel Permeation Chromatography for Molecular Weight Determinations of Plant Cell Wall Polymers: Cotton Fiber. J. Agric. Food Chem. 1991, 39, 270–275. [Google Scholar] [CrossRef]
- Triplett, B.A.; Timpa, J.D. Characterization of Cell-Wall Polymers from Cotton Ovule Culture Fiber Cells by Gel Permeation Chromatography. Vitr. Cell Dev. Biol. Plant 1995, 31, 171–175. [Google Scholar] [CrossRef]
- Kvernheim, A.L.; Lystad, E.; Jaroszewski, J.W.; Songstad, J.; Lönnberg, H.; Colacio, E.; Mulichak, A.M.; Alminger, T.; Erickson, M.; Grundevik, I.; et al. Size-Exclusion Chromatography and Methylation Analysis of Cellulose in N,N-Dimethylacetamide/LiCl. Acta Chem. Scand. 1989, 43, 209–211. [Google Scholar] [CrossRef] [Green Version]
- Marx-Figini, M.; Figini, R.V. Studies on the Mechanical Degradation of Cellulose. Die Angew. Makromol. Chem. 1995, 224, 179–189. [Google Scholar] [CrossRef]
- Jerosch, H.; Lavédrine, B.; Cherton, J.C. Study of the Stability of Cellulose-Holocellulose Solutions in N,N-Dimethylacetamide-Lithium Chloride by Size Exclusion Chromatography. J. Chromatogr. A 2001, 927, 31–38. [Google Scholar] [CrossRef]
- Nayak, J.N.; Chen, Y.; Kim, J. Removal of Impurities from Cellulose Films after Their Regeneration from Cellulose Dissolved in DMAc/LiCl Solvent System. Ind. Eng. Chem. Res. 2008, 47, 1702–1706. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wei, W.; Lickfield, G.C. Mechanical Strength of Durable Press Finished Cotton Fabric. Text. Res. J. 2000, 70, 910–915. [Google Scholar] [CrossRef]
- Gao, Q.; Shen, X.; Lu, X. Regenerated Bacterial Cellulose Fibers Prepared by the NMMO/H2O Process. Carbohydr. Polym. 2011, 83, 1253–1256. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated Cellulose by the Lyocell Process, a Brief Review of the Process and Properties. BioRes. 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Borbély, É. Lyocell, the New Generation of Regenerated Cellulose. Acta Polytech. Hung. 2008, 5, 11–18. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The Chemistry of Side Reactions and Byproduct Formation in the System NMMO/Cellulose (Lyocell Process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Perepelkin, K.E. Lyocell Fibres Based on Direct Dissolution of Cellulose in N-methylmorpholine-N-oxide: Development and Prospects. Fibre Chem. 2007, 39, 163–172. [Google Scholar] [CrossRef]
- Hudson, S.M.; Cuculo, J.A. The Solubility of Unmodified Cellulose: A Critique of the Literature. J. Macromol. Sci. Part C 1980, 18, 1–82. [Google Scholar] [CrossRef]
- Chanzy, H. Quellung Oriented Cellulose Films and Fibers from a Mesophase System. J. Polym. Sci. 1980, 18, 1137–1144. [Google Scholar] [CrossRef]
- Li, H.J.; Cao, Y.M.; Qin, J.J.; Jie, X.M.; Wang, T.H.; Liu, J.H.; Yuan, Q. Development and Characterization of Anti-Fouling Cellulose Hollow Fiber UF Membranes for Oil-Water Separation. J. Memb. Sci. 2006, 279, 328–335. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Adorjan, I.; Hofinger, A.; Sixta, H.; Firgo, H.; Kosma, P. Cellulose Solutions in N-Methylmorpholine-N-Oxide (NMMO)—Degradation Processes and Stabilizers. Cellulose 2002, 9, 283–291. [Google Scholar] [CrossRef]
- Wendler, F.; Konkin, A.; Heinze, T. Studies on the Stabilization of Modified Lyocell Solutions. Macromol. Symp. 2008, 262, 72–84. [Google Scholar] [CrossRef]
- Dogan, H.; Hilmioglu, N.D. Dissolution of Cellulose with NMMO by Microwave Heating. Carbohydr. Polym. 2009, 75, 90–94. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Mohite, L.V.; Deshmukh, N.A.; Pinjari, D.V. Effect of Ultrasound Treatment on Swelling Behavior of Cellulose in Aqueous N-Methylmorpholine-N-Oxide Solution. Ultrason. Sonochem. 2018, 49, 161–168. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic Liquids and Catalysis: Recent Progress from Knowledge to Applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Ohno, H.; Fukaya, Y. Task Specific Ionic Liquids for Cellulose Technology. Chem. Lett. 2009, 38, 2–7. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and Purification of Ionic Liquids from Solutions: A Review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [Green Version]
- Mecerreyes, D. Polymeric Ionic Liquids: Broadening the Properties and Applications of Polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Chatel, G.; Pereira, J.F.B.; Debbeti, V.; Wang, H.; Rogers, R.D. Mixing Ionic Liquids—“Simple Mixtures” or “Double Salts”? Green Chem. 2014, 16, 2051–2083. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Boeck, G.; Verevkin, S.P.; Ludwig, R. Volatile Times for the Very First Ionic Liquid: Understanding the Vapor Pressures and Enthalpies of Vaporization of Ethylammonium Nitrate. Chemistry 2014, 20, 11640–11645. [Google Scholar] [CrossRef]
- Freemantle, M. Designer Solvents. Chem. Eng. News Arch. 1998, 76, 32–37. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellulose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Fort, D.A.; Remsing, R.C.; Swatloski, R.P.; Moyna, P.; Moyna, G.; Rogers, R.D. Can Ionic Liquids Dissolve Wood? Processing and Analysis of Lignocellulosic Materials with 1-n-Butyl-3-Methylimidazolium Chloride. Green Chem. 2007, 9, 63–69. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R.D. Complete Dissolution and Partial Delignification of Wood in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Shamshina, J.L. Chitin in Ionic Liquids: Historical Insights into the Polymer’s Dissolution and Isolation. A Review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; De Long, H.C.; Mantz, R.A. Dissolution and Regeneration of Bombyx Mori Silk Fibroin Using Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef]
- Dias, R.M.; Petrin, L.C.G.; Sosa, F.B.H.; da Costa Lopes, M.; Coutinho, A.P.; da Costa, M.C. Investigation of Kraft Lignin Solubility in Protic Ionic Liquids and Their Aqueous Solutions. Ind. Eng. Chem. Res 2020, 59, 18193–18202. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Spychaj, T. Ionic Liquids: Media for Starch Dissolution, Plasticization and Modification. Carbohydr. Polym. 2011, 86, 424–428. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Liu, Y.; Zhang, S.; Skov, A.L. Screening of Ionic Liquids for Keratin Dissolution by Means of COSMO-RS and Experimental Verification. ACS Sustainable Chem. Eng. 2018, 6, 17314–17322. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a Molecular Understanding of Cellulose Dissolution in Ionic Liquids: Anion/Cation Effect, Synergistic Mechanism and Physicochemical Aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Sashina, E.S.; Kashirskii, D.A.; Busygin, K.N. Dissolution of Cellulose with Pyridinium-Based Ionic Liquids: Effect of Chemical Structure and Interaction Mechanism. Cellul. Chem. Technol. 2016, 50, 199–211. [Google Scholar]
- Elsayed, S.; Hellsten, S.; Guizani, C.; Witos, J.; Rissanen, M.; Rantamäki, A.H.; Varis, P.; Wiedmer, S.K.; Sixta, H. Recycling of Superbase-Based Ionic Liquid Solvents for the Production of Textile-Grade Regenerated Cellulose Fibers in the Lyocell Process. ACS Sustain. Chem. Eng. 2020, 8, 14217–14227. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Thakurathi, M.; Knight, M.; Quitevis, E.L.; Abidi, N. Dissolution of Cotton Cellulose in 1:1 Mixtures of 1-Butyl-3-Methylimidazolium Methylphosphonate and 1-Alkylimidazole Co-Solvents. Carbohydr. Polym. 2019, 221, 63–72. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic Liquids as Reaction Medium in Cellulose Functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Zhao, Y.; Wang, J. Cellulose Dissolution at Ambient Temperature: Role of Preferential Solvation of Cations of Ionic Liquids by a Cosolvent. Carbohydr. Polym. 2013, 92, 540–544. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Lu, X.; Zhang, X.; Zhang, S.; Zhou, K. Characterization of the Regenerated Cellulose Films in Ionic Liquids and Rheological Properties of the Solutions. Mater. Chem. Phys. 2011, 128, 220–227. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of Ionic Liquids as More Efficient Media for Cellulose Dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Production of Bioactive Cellulose Films Reconstituted from Ionic Liquids. Biomacromolecules 2004, 5, 1379–1384. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.D.; Daly, D.T.; Rogers, R.D. Ionic Liquid-Reconstituted Cellulose Composites as Solid Support Matrices for Biocatalyst Immobilization. Biomacromolecules 2005, 6, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, L.; Yu, J.; Wu, J.; Zhang, X.; He, J.; Zhang, J. Bio-Based and Biodegradable Polymers Understanding Cellulose Dissolution: Effect of the Cation and Anion Structure of Ionic Liquids on the Solubility of Cellulose. Sci. China Chem. 2016, 59, 1421–1429. [Google Scholar] [CrossRef]
- Endo, T.; Hosomi, S.; Fujii, S.; Ninomiya, K.; Takahashi, K. Anion Bridging-Induced Structural Transformation of Cellulose Dissolved in Ionic Liquid. J. Phys. Chem. Lett 2016, 7, 5156–5161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of Anionic Structure on the Dissolution of Cellulose in Ionic Liquids Revealed by Molecular Simulation. Carbohydr. Polym. 2013, 94, 723–730. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of Cellulose Dissolution in the Ionic Liquid 1-n-Butyl-3- Methylimidazolium Chloride: A 13C and 35/37Cl NMR Relaxation Study on Model Systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of Cationic Structure on Cellulose Dissolution in Ionic Liquids: A Molecular Dynamics Study. ChemPhysChem 2012, 13, 3126–3133. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The Solvatochromic Comparison Method. 2. The α-Scale of Solvent Hydrogen-Bond Donor (HBD) Acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Hopcroft, J.E.; Ullman, J.D.; Aho, A.V.; Yokoyama, T.; Taft, R.W.; Kamlet, M.J. 8—New Developments of the Graph Traverser. In Machine Intelligence; Elsevier: Amsterdam, The Netherlands, 1967; pp. 119–127. [Google Scholar]
- Yokoyama, T.; Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 3. Hydrogen bonding by some 2-nitroaniline derivatives. J. Am. Chem. Soc. 1976, 98, 3233–3237. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of Lignocellulosic Biomass with Ionic Liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. NMR Spectroscopic Studies of Cellobiose Solvation in EmimAc Aimed to Understand the Dissolution Mechanism of Cellulose in Ionic Liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, S.; Yao, Y.; Yao, X.; Xu, J.; Lu, X. Dissolving Process of a Cellulose Bunch in Ionic Liquids: A Molecular Dynamics Study. Phys. Chem. Chem. Phys. 2015, 17, 17894–17905. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z. Research Progress on Dissolution and Functional Modification of Cellulose in Ionic Liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous Dissolution of Cellulose in Organic Electrolyte Solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Insight into the Cosolvent Effect of Cellulose Dissolution in Imidazolium-Based Ionic Liquid Systems. J. Phys. Chem. B 2013, 117, 9042–9049. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Gomes, M.F.C. Understanding the Role of Co-Solvents in the Dissolution of Cellulose in Ionic Liquids. Green Chem. 2014, 16, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Velioglu, S.; Yao, X.; Devémy, J.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B.; Dequidt, A.; Costa Gomes, M.F.; Pádua, A.A.H. Solvation of a Cellulose Microfibril in Imidazolium Acetate Ionic Liquids: Effect of a Cosolvent. J. Phys. Chem. B 2014, 118, 14860–14869. [Google Scholar] [CrossRef]
- Zavgorodnya, O.; Shamshina, J.L.; Berton, P.; Rogers, R.D. Translational Research from Academia to Industry: Following the Pathway of George Washington Carver. In Ionic Liquids: Current State and Future Directions; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1250, pp. 2–17. [Google Scholar] [CrossRef]
- Tullo, A.H. The Time is Now for Ionic Liquids. Chem. Eng. News. February 3, 2020. Available online: https://0-cen-acs-org.brum.beds.ac.uk/materials/ionic-liquids/time-ionic-liquids/98/i5 (accessed on 26 November 2021).
- Kadokawa, J.-i.; Murakami, M.; Kaneko, Y. A Facile Preparation of Gel Materials from a Solution of Cellulose in Ionic Liquid. Carbohydr. Res. 2008, 343, 769–772. [Google Scholar] [CrossRef]
- Aaltonen, O.; Jauhiainen, O. The Preparation of Lignocellulosic Aerogels from Ionic Liquid Solutions. Carbohydr. Polym. 2009, 75, 125–129. [Google Scholar] [CrossRef]
- Quan, S.-L.; Kang, S.-G.; Chin, I.-J. Characterization of Cellulose Fibers Electrospun Using Ionic Liquid. Cellulose 2010, 17, 223–230. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.G.; Zhang, Z.N.; Wu, J.; Zhang, J.; He, J.S. Regenerated-Cellulose/Multiwalled- Carbon-Nanotube Composite Fibers with Enhanced Mechanical Properties Prepared with the Ionic Liquid 1-Allyl-3-Methylimidazolium Chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, B.; Li, H.S.; Li, Y.Q.; Ou, S.Y. Green Composite Films Composed of Nanocrystalline Cellulose and a Cellulose Matrix Regenerated from Functionalized Ionic Liquid Solution. Carbohydr. Polym. 2011, 84, 383–389. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–Water Separation Using Membranes Manufactured from Cellulose/Ionic Liquid Solutions. ACS Sustain. Chem. Eng. 2019, 7, 5649–5659. [Google Scholar] [CrossRef] [Green Version]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.L.; Zhou, J.; Zhang, L.L.; Kennedy, J.F. Structure and Properties of Hydrogels Prepared from Cellulose in NaOH/Urea Aqueous Solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Isobe, N.; Kim, U.J.; Kimura, S.; Wada, M.; Kuga, S. Internal Surface Polarity of Regenerated Cellulose Gel Depends on the Species Used as Coagulant. J. Colloid Interface Sci. 2011, 359, 194–201. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Matsumoto, Y.; Kuga, S. Cellulose Gel and Aerogel from LiCl/DMSO Solution. Cellulose 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Liu, P.; Zhai, M.; Li, J.; Peng, J.; Wu, J. Radiation Preparation and Swelling Behavior of Sodium Carboxymethyl Cellulose Hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Tran, T.H.; Okabe, H.; Hidaka, Y.; Hara, K. Removal of Metal Ions from Aqueous Solutions Using Carboxymethyl Cellulose/Sodium Styrene Sulfonate Gels Prepared by Radiation Grafting. Carbohydr. Polym. 2017, 157, 335–343. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of Dye by Carboxymethyl Cellulose, Acrylamide and Graphene Oxide via a Free Radical Polymerization Process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H. Adsorption of Heavy Metal Ions from Aqueous Solution onto Chitosan Entrapped CM-Cellulose Hydrogels Synthesized by Irradiation. J. Appl. Polym. Sci. 2008, 110, 1388–1395. [Google Scholar] [CrossRef]
- Kono, H. Characterization and Properties of Carboxymethyl Cellulose Hydrogels Crosslinked by Polyethylene Glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic Cellulose Hydrogels: Kinetics of the Cross-Linking Process and Characterization as pH/Ion-Sensitive Drug Delivery Systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and Swelling Behaviors of Sodium Carboxymethyl Cellulose-g-Poly(AA-Co-AM-Co-AMPS)/MMT Superabsorbent Hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Wu, C.; Guo, B.; Luo, Z. Fabrication of Mechanically Tough and Self-Recoverable Nanocomposite Hydrogels from Polyacrylamide Grafted Cellulose Nanocrystal and Poly(Acrylic Acid). Carbohydr. Polym. 2018, 198, 1–8. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kuga, S. Polyallylamine-Grafted Cellulose Gel as High-Capacity Anion-Exchanger. J. Chromatogr. A 2002, 946, 283–289. [Google Scholar] [CrossRef]
- Aulin, C.; Netrval, J.; Wågberg, L.; Lindström, T. Aerogels from Nanofibrillated Cellulose with Tunable Oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Potter, O.G.; Hilder, E.F. Porous Polymer Monoliths for Extraction: Diverse Applications and Platforms. J. Sep. Sci. 2008, 31, 1881–1906. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Jackson, T.; Moussa, H.; Abidi, N. Preparation, Characterization, and Cationic Functionalization of Cellulose-Based Aerogels for Wastewater Clarification. J. Mater. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kistler, S.S. Coherent Expanded Aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Tan, C.; Fung, B.M.; Newman, J.K.; Vu, C. Organic Aerogels with Very High Impact Strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Parajuli, P.; Acharya, S.; Hu, Y.; Abidi, N. Cellulose-based Monoliths with Enhanced Surface Area and Porosity. J. Appl. Polym. Sci. 2020, 137, 1–12. [Google Scholar] [CrossRef]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose Aerogels Prepared from an Aqueous Zinc Chloride Salt Hydrate Melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef]
- Innerlohinger, J.; Weber, H.K.; Kraft, G. Aerocellulose: Aerogels and Aerogel-like Materials Made from Cellulose. Macromol. Symp. 2006, 244, 126–135. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of Hierarchical Porous Structures on the Mechanical Properties of Cellulose Aerogels. J. Sol-Gel Sci. Technol. 2019, 89, 156–165. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Wang, Y.; Zhao, J.; Zhang, W.; Lu, C. Aerogels from Quaternary Ammonium-Functionalized Cellulose Nanofibers for Rapid Removal of Cr(VI) from Water. Carbohydr. Polym. 2014, 111, 683–687. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Nie, J.; Yang, J.; Qi, X.; Zhou, Z.; Wang, Y. Bio-Inspired Functionalization of Microcrystalline Cellulose Aerogel with High Adsorption Performance toward Dyes. Carbohydr. Polym. 2018, 198, 546–555. [Google Scholar] [CrossRef]
- Panzella, L.; Melone, L.; Pezzella, A.; Rossi, B.; Pastori, N.; Perfetti, M.; D’Errico, G.; Punta, C.; D’Ischia, M. Surface-Functionalization of Nanostructured Cellulose Aerogels by Solid State Eumelanin Coating. Biomacromolecules 2016, 17, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, J. Cellulose Aerogels Functionalized with Polypyrrole and Silver Nanoparticles: In-Situ Synthesis, Characterization and Antibacterial Activity. Carbohydr. Polym. 2016, 146, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.P.; Greenberg, A.R.; Kelley, S.S.; Pilath, H.; Juhn Roh, I.; Tyber, J. Synthesis and Characterization of Dense and Porous Cellulose Films. J. Appl. Polym. Sci. 2007, 105, 1228–1236. [Google Scholar] [CrossRef]
- Cai, J.; Wang, L.; Zhang, L. Influence of Coagulation Temperature on Pore Size and Properties of Cellulose Membranes Prepared from NaOH–Urea Aqueous Solution. Cellulose 2007, 14, 205–215. [Google Scholar] [CrossRef]
- Yang, Q.; Fukuzumi, H.; Saito, T.; Isogai, A.; Zhang, L. Transparent Cellulose Films with High Gas Barrier Properties Fabricated from Aqueous Alkali/Urea Solutions. Biomacromolecules 2011, 12, 2766–2771. [Google Scholar] [CrossRef]
- Jia, B.; Mei, Y.; Cheng, L.; Zhou, J.; Zhang, L. Preparation of Copper Nanoparticles Coated Cellulose Films with Antibacterial Properties through One-Step Reduction. ACS Appl. Mater. Interfaces 2012, 4, 2897–2902. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Xu, M. Novel Regenerated Cellulose Films Prepared by Coagulating with Water: Structure and Properties. Carbohydr. Polym. 2012, 87, 95–100. [Google Scholar] [CrossRef]
- Garnier, G.; Wright, J.; Godbout, L.; Yu, L. Wetting Mechanism of Alkyl Ketene Dimers on Cellulose Films. Colloids Surf. A Physicochem. Eng. Asp. 1998, 145, 153–165. [Google Scholar] [CrossRef]
- Da Róz, A.L.; Leite, F.L.; Pereiro, L.V.; Nascente, P.A.P.; Zucolotto, V.; Oliveira, O.N.; Carvalho, A.J.F. Adsorption of Chitosan on Spin-Coated Cellulose Films. Carbohydr. Polym. 2010, 80, 65–70. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and Regeneration of Cellulose in NaOH/Thiourea Aqueous Solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A Bioplastic with High Strength Constructed from a Cellulose Hydrogel by Changing the Aggregated Structure. J. Mater. Chem. A 2013, 1, 6678–6686. [Google Scholar] [CrossRef]
- Takegawa, A.; Murakami, M.; Kaneko, Y.; Kadokawa, J. Preparation of Chitin/Cellulose Composite Gels and Films with Ionic Liquids. Carbohydr. Polym. 2010, 79, 85–90. [Google Scholar] [CrossRef]
- Dahlan, N.A.; Veeramachineni, A.K.; Langford, S.J.; Pushpamalar, J. Developing of a Magnetite Film of Carboxymethyl Cellulose Grafted Carboxymethyl Polyvinyl Alcohol (CMC-g-CMPVA) for Copper Removal. Carbohydr. Polym. 2017, 173, 619–630. [Google Scholar] [CrossRef]
- Liew, S.Y.; Thielemans, W.; Walsh, D.A. Electrochemical Capacitance of Nanocomposite Polypyrrole/Cellulose Films. J. Phys. Chem. C 2010, 114, 17926–17933. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Kafy, A.; Kim, H.-C.; Ko, H.-U.; Mun, S.; Kim, J. Reduced Graphene Oxide Filled Cellulose Films for Flexible Temperature Sensor Application. Synth. Methods 2015, 206, 154–161. [Google Scholar] [CrossRef]
- Sievens-Figueroa, L.; Bhakay, A.; Jerez-Rozo, J.I.; Pandya, N.; Romañach, R.J.; Michniak-Kohn, B.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Preparation and Characterization of Hydroxypropyl Methyl Cellulose Films Containing Stable BCS Class II Drug Nanoparticles for Pharmaceutical Applications. Int. J. Pharm. 2012, 423, 496–508. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and Oil Barrier Properties of Microfibrillated Cellulose Films and Coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and Forming of Cellulose with Ionic Liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Michud, A.; Sixta, H. Dry Jet-Wet Spinning of Strong Cellulose Filaments from Ionic Liquid Solution. Cellulose 2014, 21, 4471–4481. [Google Scholar] [CrossRef]
- Fu, F.; Yang, Q.; Zhou, J.; Hu, H.; Jia, B.; Zhang, L. Structure and Properties of Regenerated Cellulose Filaments Prepared from Cellulose Carbamate–NaOH/ZnO Aqueous Solution. ACS Sustain. Chem. Eng. 2014, 2, 2604–2612. [Google Scholar] [CrossRef]
- Frenot, A.; Henriksson, M.W.; Walkenström, P. Electrospinning of Cellulose-Based Nanofibers. J. Appl. Polym. Sci. 2007, 103, 1473–1482. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of Ultrafine Cellulose Acetate Fibers: Studies of a New Solvent System and Deacetylation of Ultrafine Cellulose Acetate Fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Freire, M.; Teles, A.; Lopes-da-Silva, J.; Coutinho, J. Electrospinning of Cellulose in Ionic Liquids Solutions. In Proceedings of the 239th ACS National Meeting, San Francisco, CA, USA, 21–25 March 2010. [Google Scholar]
- Freire, M.G.; Teles, A.R.R.; Ferreira, R.A.S.; Carlos, L.D.; Lopes-da-Silva, J.A.; Coutinho, J.A.P. Electrospun Nanosized Cellulose Fibers Using Ionic Liquids at Room Temperature. Green Chem. 2011, 13, 3173–3180. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, G.; Murugesan, S.; Pushparaj, V.; Nalamasu, O.; Ajayan, P.M.; Linhardt, R.J. Preparation of Biopolymer Fibers by Electrospinning from Room Temperature Ionic Liquids. Biomacromolecules 2006, 7, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Linhardt, R.; Murugesan, S. Cellulose Composites Prepared Using Room Temperature Ionic Liquids. In Proceedings of the 233rd ACS National Meeting, Chicago, IL, USA, 25–29 March 2007. [Google Scholar]
- Polaskova, M.; Cermak, R.; Verney, V.; Ponizil, P.; Commereuc, S.; Gomes, M.F.C.; Padua, A.A.H.; Mokrejs, P.; MacHovsky, M. Preparation of Microfibers from Wood/Ionic Liquid Solutions. Carbohydr. Polym. 2013, 92, 214–217. [Google Scholar] [CrossRef]
- Kang, Y.; Ahn, Y.; Lee, S.H.; Hong, J.H.; Ku, M.K.; Kim, H. Lignocellulosic Nanofiber Prepared by Alkali Treatment and Electrospinning Using Ionic Liquid. Fibers Polym. 2013, 14, 530–536. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.H.; Kim, H.J.; Yang, Y.H.; Hong, J.H.; Kim, Y.H.; Kim, H. Electrospinning of Lignocellulosic Biomass Using Ionic Liquid. Carbohydr. Polym. 2012, 88, 395–398. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; He, A.; Li, J.; Zhang, H.; Han, C.C. Electrospinning of Native Cellulose from Nonvolatile Solvent System. Polymer 2008, 49, 2911–2917. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional Cellulose-Based Hydrogels for Biomedical Applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a Bacterial Cellulose-Based Hydrogel Cell Carrier Containing Keratinocytes and Fibroblasts for Full-Thickness Wound Healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef] [Green Version]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of Nanocellulose Scaffolds with Tunable Structures to Support 3D Cell Culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-Based Scaffold Materials for Cartilage Tissue Engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Tassin, J.-F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite Nanoparticle-Associated Silated Hydroxypropylmethyl Cellulose as an Injectable Reinforced Interpenetrating Network Hydrogel for Cartilage Tissue Engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Pierron, D.; Harmand, M.-F. Fibrous Cellulose Nanocomposite Scaffolds Prepared by Partial Dissolution for Potential Use as Ligament or Tendon Substitutes. Carbohydr. Polym. 2012, 87, 2291–2298. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Appl. Mater. Interfaces 2017, 9, 34314–34324. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose Nanocrystals and Cellulose Nanofibrils Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.S.; Gunathilake, C.; Jackson, T.; Jaroniec, M.; Abidi, N. Preparation and Adsorption Properties of Aerocellulose-Derived Activated Carbon Monoliths. Cellulose 2016, 23, 1–12. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose Aerogel from Paper Waste for Crude Oil Spill Cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Tan, Y.; Du, J.; Wang, J. Synthesis and Characterization of a Porous and Hydrophobic Cellulose-Based Composite for Efficient and Fast Oil-Water Separation. Carbohydr. Polym. 2016, 140, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu(Ii), Zn(Ii), and Pb(Ii) from Aqueous Single and Binary Metal Solutions by Regenerated Cellulose and Sodium Alginate Chemically Modified with Polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan(Chitin)/Cellulose Composite Biosorbents Prepared Using Ionic Liquid for Heavy Metal Ions Adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Tan, W.Z.; Liu, X.H.; Sun, Z.F.; Ren, P.G.; Yan, D.X. Facile Preparation of 3D Regenerated Cellulose/Graphene Oxide Composite Aerogel with High-Efficiency Adsorption towards Methylene Blue. J. Colloid Interface Sci. 2018, 532, 58–67. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/Silk Fibroin Assisted Calcium Phosphate Growth: Novel Biocomposite for Dye Adsorption. Int. J. Biol. Macromol. 2020, 165, 1970–1977. [Google Scholar] [CrossRef]
- Ma, X.; Deng, Q.; Wang, L.; Zheng, X.; Wang, S.; Wang, Q.; Chen, L.; Huang, L.; Ouyang, X.; Cao, S. Cellulose Transparent Conductive Film and Its Feasible Use in Perovskite Solar Cells. RSC Adv. 2019, 9, 9348–9353. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, H.; Huang, K.; Cao, S.; Ni, Y.; Huang, L.; Chen, L.; Ouyang, X. Conductive Regenerated Cellulose Film as Counter Electrode for Efficient Dye-Sensitized Solar Cells. Cellulose 2018, 25, 5113–5122. [Google Scholar] [CrossRef]
- Ebner, M.; Schennach, R.; Chien, H.T.; Mayrhofer, C.; Zankel, A.; Friedel, B. Regenerated Cellulose Fiber Solar Cell. Flex. Print. Electron. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Chen, W.; Yi, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Li, X.; Yu, H. Highly Flexible and Conductive Cellulose-Mediated PEDOT:PSS/MWCNT Composite Films for Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 13213–13222. [Google Scholar] [CrossRef]
- Liu, S.; He, K.; Wu, X.; Luo, X.; Li, B. Surface Modification of Cellulose Scaffold with Polypyrrole for the Fabrication of Flexible Supercapacitor Electrode with Enhanced Capacitance. RSC Adv. 2015, 5, 87266–87276. [Google Scholar] [CrossRef]
- Pasta, M.; la Mantia, F.; Hu, L.; Deshazer, H.D.; Cui, Y. Aqueous Supercapacitors on Conductive Cotton. Nano Res. 2010, 3, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Nyström, G.; Strømme, M.; Sjödin, M.; Nyholm, L. Rapid Potential Step Charging of Paper-Based Polypyrrole Energy Storage Devices. Electrochim. Acta 2012, 70, 91–97. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Sevilla, M.; Ferrero, G.A.; Fuertes, A.B. Graphene-Cellulose Tissue Composites for High Power Supercapacitors. Energy Storage Mater. 2016, 5, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-Based Supercapacitors for Self-Powered Nanosystems. Angew. Chem. Int. Ed. 2012, 51, 4934–4938. [Google Scholar] [CrossRef]
- Miller, J.R.; Burke, A.F. Electrochemical Capacitors: Challenges and Opportunities for Real-World Applications. Electrochem. Soc. Interface 2008, 17, 53–57. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-Based Li-Ion Batteries: A Review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional Hydrogels for Next-Generation Batteries and Supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yu, J.; Zhang, J. Cellulose Aerogel Membranes with a Tunable Nanoporous Network as a Matrix of Gel Polymer Electrolytes for Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 24591–24599. [Google Scholar] [CrossRef]
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Tong, S.; He, Z.; Yang, R. Recent Developments of Cellulose Materials for Lithium-Ion Battery Separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Xiao, S.Y.; Li, M.X.; Chang, Z.; Wang, F.X.; Gao, J.; Wu, Y.P. Natural Macromolecule Based Carboxymethyl Cellulose as a Gel Polymer Electrolyte with Adjustable Porosity for Lithium Ion Batteries. J. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A Review of Recent Developments in Membrane Separators for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Li, S.; Ren, G.; Hoque, M.N.F.; Dong, Z.; Warzywoda, J.; Fan, Z. Carbonized Cellulose Paper as an Effective Interlayer in Lithium-Sulfur Batteries. Appl. Surf. Sci. 2017, 396, 637–643. [Google Scholar] [CrossRef]
- Wang, Z.; Malti, A.; Ouyang, L.; Tu, D.; Tian, W.; Wågberg, L.; Hamedi, M.M. Copper-Plated Paper for High-Performance Lithium-Ion Batteries. Small 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Wang, X.; Liu, R.; Zhang, J.; Yue, L.; Duan, Y.; Cui, G. Cellulose/Polysulfonamide Composite Membrane as a High Performance Lithium-Ion Battery Separator. ACS Sustain. Chem. Eng. 2014, 2, 194–199. [Google Scholar] [CrossRef]
- Illera, D.; Mesa, J.; Gomez, H.; Maury, H. Cellulose Aerogels for Thermal Insulation in Buildings: Trends and Challenges. Coatings 2018, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z.; Wang, Y.Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 8, 623. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.T.; Feng, J.; Ng, S.K.; Wong, J.P.W.; Tan, V.B.C.; Duong, H.M. Advanced Thermal Insulation and Absorption Properties of Recycled Cellulose Aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Karadagli, I.; Schulz, B.; Schestakow, M.; Milow, B.; Gries, T.; Ratke, L. Production of Porous Cellulose Aerogel Fibers by an Extrusion Process. J. Supercrit. Fluids 2015, 106, 105–114. [Google Scholar] [CrossRef]
- Vargaftik, N.B. Handbook of Thermal Conductivity of Liquids and Gases; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Lu, Y.; Sun, Q.; Yang, D.; She, X.; Yao, X.; Zhu, G.; Liu, Y.; Zhao, H.; Li, J. Fabrication of Mesoporous Lignocellulose Aerogels from Wood via Cyclic Liquid Nitrogen Freezing-Thawing in Ionic Liquid Solution. J. Mater. Chem. 2012, 22, 13548–13557. [Google Scholar] [CrossRef]
- Sequeira, S.; Evtuguin, D.V.; Portugal, I. Preparation and Properties of Cellulose/Silica Hybrid Composites. Polym. Polym. Compos. 2009, 30, 1275–1282. [Google Scholar] [CrossRef]
- Shi, J.; Lu, L.; Guo, W.; Liu, M.; Cao, Y. On Preparation, Structure and Performance of High Porosity Bulk Cellulose Aerogel. Plast. Rubber Compos. 2015, 44, 26–32. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Xu, D.; Yang, Y. A Facile Method to Prepare Cellulose Whiskers–Silica Aerogel Composites. J. Sol-Gel Sci. Technol. 2017, 83, 72–80. [Google Scholar] [CrossRef]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.S.; Xia, H.; Gong, S. Poly(Vinyl Alcohol)/Cellulose Nanofibril Hybrid Aerogels with an Aligned Microtubular Porous Structure and Their Composites with Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from Waste Cotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Yu, J.; Song, R.; Mi, Q.; He, J.; Zhang, J. Transparent and Flame Retardant Cellulose/Aluminum Hydroxide Nanocomposite Aerogels. Sci. China Chem. 2016, 59, 1335–1341. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward Aerogel Based Thermal Superinsulation in Buildings: A Comprehensive Review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose—Silica Aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef]
- Laskowski, J.; Milow, B.; Ratke, L. The Effect of Embedding Highly Insulating Granular Aerogel in Cellulosic Aerogel. J. Supercrit. Fluids 2015, 106, 93–99. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose-Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chem. 2012, 124, 2118–2121. [Google Scholar] [CrossRef]
- Armir, N.A.Z.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Othman, M.H.C.; Zakaria, S. Regenerated cellulose products for agricultural and their potential: A review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef]
- Friuli, M.; Nitti, P.; Aneke, C.I.; Demitri, C.; Cafarchia, C.; Otranto, D. Freeze-drying of Beauveria bassiana suspended in Hydroxyethyl cellulose based hydrogel as possible method for storage: Evaluation of survival, growth and stability of conidial concentration before and after processing. Results Eng. 2021, 12, 100283. [Google Scholar] [CrossRef]
- Friuli, M.; Nitti, P.; Cafuero, L.; Prete, A.; Zafar, M.S.; Madaghiele, M.; Demitri, C. Cellulose Acetate and Cardanol Based Seed Coating for Intraspecific Weeding Coupled with Natural Herbicide Spraying. J. Polym. Environ. 2020, 28, 2893–2904. [Google Scholar] [CrossRef]






| Solvent System | Cellulose Source | Pretreatment | Effects on Cellulose | Dissolution Conditions | Ref. | ||
|---|---|---|---|---|---|---|---|
| Conc. (wt%) | Temp. (°C) | Time (h) | |||||
| NaOH/H2O | |||||||
| NaOH/H2O (9.1:90.9 w/w) | Soft wood and hard wood pulps, DP 1060; 994 | Steam explosion | Decrease DP DP 180; 287 | 5 | 4 | 8 | [82] |
| NaOH/H2O (8:92 w/w) | Dissolving sulfite wood pulps; DP 2375; 1410 | Enzymatic (Cellulase + Econase HC400) | Digestion of primary cell wall, Decrease DP DP 925; 590 | 0.7 a | −6 | 2 | [85] |
| NaOH/H2O (7.6:92.4 w/w) | Commercial MCC; DP ~170 | None | NA | 7.6 | −6 | Footnote b | [66] |
| NaOH/Urea/H2O | |||||||
| NaOH/urea/H2O (7:12:81 w/w) | Commercial softwood unbleached Kraft pulp; DP 1300 | Ball milling | Decrease DP and crystallinity DP 330, CrI ~0% | 1 | −12 | >24 | [86] |
| NaOH/Urea/H2O (7:12:81 w/w) | Commercial dissolving pulp; DP ~750 | Ethanol–hydrochloric acid | Decrease DP, weakening cell wall DP ~190 | 4 | −10 | >2 | [47] |
| NaOH/Urea/H2O (6:4:90 w/w) | Cotton linters; DP 850 | Enzymatic (Celluclast) | Decrease DP DP 620–680 | 1.2 c | −15 | 4 | [84] |
| NaOH/Urea/H2O (6:4:90 w/w) | Cotton linters; DP ~410 | None | NA | 5 | 0 | 12 | [77] |
| NaOH/Urea/H2O (7:12:81 w/w) | Cotton linters; DP 570 | None | NA | 4 | 0 | Footnote d | [87] |
| NaOH/Urea/H2O (7:12:81 w/w) | Cotton linters; DP ~620 | None | NA | 4 | −10 | 0.1 | [88] |
| NaOH/Urea/ZnO/H2O | |||||||
| NaOH/Urea/ ZnO/H2O (10.2:4.2:0.8:84.8 w/w) | Commercial cellulose pulp, DP 553 | Hydrothermal | Decrease DP, decrease polydispersity (PDI) DP 290–405 | 7.45 | 2 | 0.2 | [81] |
| NaOH/Urea/ ZnO/H2O (7:12:0.5:80.5 w/w) | Commercial cotton linter pulp; DP ~1050 | None | NA | 2.5 | −13 | 2 | [89] |
| NaOH/Urea/Thiourea/H2O | |||||||
| NaOH/Urea/ Thiourea/H2O (8:8:6:78 w/w) | Dissolving pulps; DP 780 | Steam explosion | Decrease DP DP ~150 | 5 | −10 | 60 Footnote e | [90] |
| NaOH/Thiourea/ H2O (9.5:4.5:86 w/w) | Pulp sheets of cotton linters; DP 330; 620 | None | NA | 5, 7.5 | −8; −10 | 0.1 | [91] |
| Other Systems | |||||||
| NaOH/PEG/H2O (9:1:90 w/w) | Commercial cellulose powder; DP ~810 | None | NA | 13 | −15 | 15 | [92] |
| NaOH/TMAH/H2O (9.2:21.0:69.8 w/w) f | Commercial MCC; DP ~180 | None | NA | 5 | −20 | Footnote g | [93] |
| Solvent Systems | Cellulose (Mw) or Degree of Polymerization (DP) | Load, wt.% | Dissolution Conditions | Ref. |
|---|---|---|---|---|
| [C4mim]Cl | Cellulose pulp (DP ≈ 1000) | 10 | Thermal dissolution at 100 °C | [150] |
| [C4mim]Cl | Cellulose pulp (DP ≈ 1000) | 3 | Thermal dissolution at 70 °C | [150] |
| [C4mim]Cl | Cellulose pulp (DP ≈ 1000) | 5 | Thermal dissolution at 80 °C + sonication | [150] |
| [C4mim]Cl | Cellulose pulp (DP ≈ 1000) | 25 | Microwave-assisted dissolution (at 100–130 °C) for a few minutes in 3–5 s pulses | [150] |
| [C4mim]Br | Cellulose pulp (DP ≈ 1000) | 5–7 | Microwave-assisted dissolution (at 100–130 °C) for a few minutes in 3–5 s pulses | [150] |
| [C4mim][SCN] | Cellulose pulp (DP ≈ 1000) | 5–7 | Microwave-assisted dissolution (at 100–130 °C) for a few minutes in 3–5 s pulses | [150] |
| [C6mim]Cl | Cellulose pulp (DP ≈ 1000) | 5 | Thermal dissolution at 100 °C | [150] |
| [C2C1im][(OMe)(H)PO2] with 1-alkylimidazoles (Alkyl = CH3, C2H5, C3H7, C4H9) | Scoured, bleached, air-dried, ground cotton fibers | 5 | Microwave-assisted dissolution followed by 90 °C oven: dissolved immediately after microwave or stored in oven up to 1 h | [162] |
| [C4C1im][(OMe)(H)PO2] with 1-alkylimidazoles (Alkyl = CH3, C2H5, C3H7, C4H9) | Scoured, bleached, air-dried, ground cotton fibers | 5 | Microwave-assisted dissolution followed by 90 °C oven: dissolved immediately after microwave or stored in oven up to 1 h | [162] |
| [(Bnz)2im][OAc] (Bnz = benzyl) | Scoured, bleached, air-dried, ground cotton fibers | 5 | Microwave-assisted dissolution followed by 90 °C oven: dissolved immediately after microwave or stored in oven up to 1 h | [40] |
| [NapmC1im][OAc] (Nap = Naphtalyl) | Scoured, bleached, air-dried, ground cotton fibers | 5 | Microwave-assisted dissolution followed by 90 °C oven: dissolved immediately after microwave or stored in oven up to 1 h | [40] |
| [C4mim]Cl | Avicel (DP 286), spruce sulfite pulp (dp-593), cotton Linters (dp-1198) | 1–39 | Thermal dissolution at 80 °C, 12 h | [163] |
| [C4mim][CH3COO]/DMSO = 2.54:1 mol/mol | MCC (DP 229) | 15 | Not provided | [164] |
| [C4mim][PhCOO]/DMSO = 2.54:1 mol/mol | MCC (DP 229) | 9 | Not provided | [164] |
| [C2mim][OAc] | Cotton pulp (the α-cellulose content 94%, DP 510) | 16 | 90 °C oil bath for 7 h | [165] |
| [C4mim][OAc] | Cotton pulp (the α-cellulose content 94%, DP 510) | 15 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C2mim]Cl | Cotton pulp (the α-cellulose content 94%, DP 510) | 14 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C4mim]Cl | Cotton pulp (the α-cellulose content 94%, DP 510) | 13 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C4mim][HSO4] | Cotton pulp (the α-cellulose content 94%, DP 510) | 11 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C4mim][FeCl4] | Cotton pulp (the α-cellulose content 94%, DP 510) | <5 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C4mim]Br | Cotton pulp (the α-cellulose content 94%, DP 510) | <3 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C2mim]Br | Cotton pulp (the α-cellulose content 94%, DP 510) | <5 | Thermal dissolution at 70 °C for 7 h | [165] |
| [C2mim]Cl/[C2mim][OAc] = 30:70 mol/mol | MCC | 40 | Stepwise thermal dissolution of cellulose at 100 °C to determine solubility | [166] |
| [C2mim]Cl/[C4mim]Cl eutectic mixture | MCC | 35 | Stepwise thermal dissolution of cellulose at 100 °C to determine solubility | [166] |
| [C2mim]Cl | MCC | 12 | Stepwise thermal dissolution of cellulose at 100 °C to determine solubility | [166] |
| [C4mim]Cl | MCC | 29 | Stepwise thermal dissolution of cellulose at 100 °C to determine solubility | [166] |
| [C2mim][OAc] | MCC | 23 | Stepwise thermal dissolution of cellulose at 100 °C to determine solubility | [166] |
| [C4mim]Cl | MCC | 4.75 | Microwave-assisted dissolution for a few minutes in 3–5 s pulses | [167] |
| [Amim]Cl | MCC | 15 | Thermal dissolution at 100 °C for 24 h | [187] |
| [C4mim]Cl | Bleached and dried softwood Kraft pulp sheets from a pulp mill | 3–7.4 | Thermal dissolution at 130 °C for 4 h | [188] |
| [C4mim]Cl | Cellulose powder (MW: 194,400) | 1.5–4 | Thermal dissolution at 80 °C for up to 40 min | [189] |
| [C4mim]Cl/DMSO | Cellulose powder (MW: 194,400) | 4–5 | Thermal dissolution at 80 °C for up to 40 min | [189] |
| [Amim]Cl | Wood pulp (α-cellulose 94.9%) | 4 | Thermal dissolution at 100 °C for ~45 min | [190] |
| [C6mim]Cl | Nanocrystalline cellulose | 3 | Thermal dissolution at 85 °C for 2 h | [191] |
| [C2mim][OAc] | Avicel PH-101 microcrystalline cellulose (MW = 160,000–560,000 g/mol) | 2; 5 | Thermal dissolution at 60 °C for 1 h | [192] |
| [mTBDH][OAc] | Cellulose of birch prehydrolysis kraft pulp (Enocell) | 13 | Thermal dissolution at a temperature of 85 and 80 °C and 15 mbar with stirring (30 rpm) for 75 min | [161] |
| [DBNH][OAc] | Cellulose of birch prehydrolysis kraft pulp (Enocell) | 13 | Thermal dissolution at a temperature of 85 and 80 °C and 15 mbar with stirring (30 rpm) for 75 min | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244344
Acharya S, Liyanage S, Parajuli P, Rumi SS, Shamshina JL, Abidi N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers. 2021; 13(24):4344. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244344
Chicago/Turabian StyleAcharya, Sanjit, Sumedha Liyanage, Prakash Parajuli, Shaida Sultana Rumi, Julia L. Shamshina, and Noureddine Abidi. 2021. "Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications" Polymers 13, no. 24: 4344. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13244344