3.1. Surface Chemistry of Plasma-Treated PP
All the applied treatment conditions resulted in the incorporation of oxygen and nitrogen functional groups onto the treated PP surface (
Table 2). The concentration of oxygen functional groups was always higher than the concentration of nitrogen groups due to oxygen gas species (mainly O and OH radicals) having significantly higher reactivity with the PP chains [
26]. For the samples modified at 100 mm/s with two passes, the oxygen content was 10–11 at.% (samples denoted with
f in
Table 2). Nitrogen uptake ranged from trace amounts (samples modified using Ar and Ar/O
gas feeds) to 1.6 at.% (the sample treated in Ar/N
gas mixture at the optimized working conditions). By lowering the movement speed to 22 mm/s and increasing the number of passes to ten (samples marked with
s in
Table 2), we further increased oxygen concentration to 20–27 at.% and nitrogen concentration up to 8 at.%.
The effect of the gas feed composition on the overall concentration of functional groups attached to the treated PP samples was observable only at higher treatment times (samples denoted with
s). Of the three tested gas feeds, the PP surface treated in the Ar/O
gas mixture contained the lowest concentration of functional groups. The lower efficiency of the Ar/O
plasma treatment was most likely due to the electronegative character of oxygen plasma. Electrons sustaining the discharge were lost in attachment processes, resulting in the Ar/O
plasma having a lower electron density than a pure Ar discharge [
27]. The lower electron density resulted in lower reactive species concentrations as the reactive species are direct or indirect products of electron impact reactions [
28]. Although the addition of N
into the Ar plasma also reduces electron density [
29], the decrease in the number of functional groups attached to the PP surface was minor due to the presence of the Ar/N
postdischarge (also known as afterglow). Nitrogen afterglow is a diffuse region of a weakly ionized or neutral medium that is no longer considered plasma. In this region, reaction kinetics are no longer driven by electrons but by nitrogen species, mainly nitrogen atoms N and nitrogen metastables N
(A) [
29,
30]. Nitrogen atoms N, the most abundant species in the afterglow [
31], can subsequently react with PP [
26] resulting in the attachment of nitrogen functional groups. To summarize, the higher concentration of N in the Ar/N
postdischarge caused an increase in the probability of the reaction between N atoms and PP, resulting in higher nitrogen uptake on treated PP surface. In contrast, oxygen uptake was lower in the Ar/N
discharge as the lower plasma electron density resulted in a decrease in the concentration of reactive oxygen species. This behavior is reflected in the atomic constitution of the surface of the ArN-
s sample.
The assessment of carbon bonds on the PP surface was based on fitting the XPS C 1s signal, as described in our previous article [
21]. The four components of C 1s (
Figure 2a) were the following:
C–C/
CH
(285.0 eV),
C–O/–
C–N (287.0 ± 0.1) eV,
C=O/N–
C=O/N=
C (288.2 ± 0.1) eV, and O=
C–O (289.5 ± 0.1) eV. The
C–C/
CH
asymmetric peak shape was derived from the spectrum of pure PP. Its full width at half maximum (FWHM) was (1.05 ± 0.02) eV. The other three components were G–L shaped with the fixed G–L percentage of 30%, G–L(30) and had an FWHM of (1.30 ± 0.02) eV. Both, O 1s and N 1s, high-resolution spectra (
Figure 2b,c) were fitted by two G–L(30) components. The positions and bonds were assigned according to the available literature [
32,
33,
34]. The O 1s environment components were
O=C (532.8 ± 0.1) eV and
O–C (534.1 ± 0.1) eV. The FWHM of O 1s components was (1.78 ± 0.03) eV. The N 1s environment was composed of
N–C/
N=C (400.6 ± 0.1) eV and
N–O–C/C=
N–O (402.1 ± 0.1) eV peaks. The FWHM of N 1s components was (1.73 ± 0.05) eV.
For all the analyzed samples, the agreement between the concentration of the C1s environment components (
Figure 3) and the measured atomic percentages (
Table 2) was good. At the higher treatment speed of 100 mm/s (samples denoted with
f), the effect of plasma gas composition on the functional groups’ concentration was minor. The only notable difference was the higher number of amine and imine (
N–C/
N=C) groups at the PP surfaces treated in the Ar/N
plasma. The increase was due to the reactive nitrogen species-rich afterglow interacting with the PP surface. At 22 mm/s (samples denoted with
s), the functional group concentrations varied with the gas feed. In the following discussion, sample Ar-
s (PP treated in pure Ar PSJ) is taken as a base for the comparison. The surface of PP treated in Ar/O
plasma (sample ArO-
s) contained primarily oxygen functional groups for which its concentration ratio was similar to the Ar-
s sample. The number of nitrogen functional groups was much lower at the ArO-
s surface, and most of them were in an oxidized state. In contrast, for the Ar-
s sample, the concentration of oxidized and non-oxidized nitrogen functional groups was similar. The low concentration of nitrogen functionalities on the ArO-
s surface can be explained by how the gases were introduced into the discharge. Oxygen gas was introduced directly into the device intermixed with the main Ar flow. Therefore, most of the energy stored in the plasma—in the form of electrons, argon metastables, etc.—was used to create reactive oxygen species (O, O
, and O
). Nitrogen was introduced into the jet further downstream as an impurity coming from the surrounding atmosphere. In this region, it is reasonable to assume a low concentration of energetic species as the majority had been used previously in the reactions with oxygen. As a result, only a handful of reactive nitrogen gas species are generated; hence, the low number of nitrogen functional groups attached to the treated PP surface. The oxygen-rich plasma environment then causes their predominant oxidation state.
The addition of N
had a much larger impact on the concentration of functional groups attached to the treated PP surface (sample ArN-
s in
Figure 3) than the addition of O
. Not only the concentration of nitrogen functionalities increase, as one would expect, but the addition of N
influenced the composition of oxygen functionalities, mainly the
O–C bonds, as well. As the majority of the nitrogen functionalities is present in the form of amine or imine (
N–C/
N=C) groups, and the concentration of
O–C (respectively, O=
C–O) bonds decreased, it appears that nitrogen (more abundant) and oxygen (more reactive [
26]) gas species compete for mainly the carbon radicals. Considering a PP chain backbone scission, the changes in the functional group concentrations can be explained by two fundamentally different reaction pathways:
Nitrogen atoms react with the PP alkyl radicals;
Nitrogen atoms react with carbonyl radicals (created by the abstraction of H from the aldehyde groups (3), products of the fi-scission of secondary alkoxy radicals (2)), terminating the PP chain by the amide group (4).
In reaction (1), nitrogen ends up using the carbon radical created by the abstraction of H atoms from the PP backbone, limiting the number of sites where alkoxy radicals (the primary source of the chain backbone scission) could be formed. As a result, the ablation of PP and the formation of low molecular weight oxidized materials (LMWOMs) are reduced. On the other hand, in the reaction pathway (2)–(4), the nitrogen functional group is attached to the end of the ablated shorter PP chain, meaning that it is a part of low molecular weight oxidized materials (LMWOMs). Regarding reactivity, mid-chain alkyl radicals reactivity is much lower than the reactivity of the end-chains radical, making the second reaction pathway more likely. The results of water washing experiments, discussed in detail in
Section 3.3, show that most of the nitrogen functionalities are a part of LMWOMs, confirming the reactivity-based assumption. To summarize the effect of the addition of N
to the Ar gas feed on the functional group composition, the observed decrease in the concentrations of
O–C and O=
C–C environments was not caused by the lower degree of the PP chain scission but by a portion of the carboxyl terminating groups being replaced with amides.
As shown in
Figure 4a,d, the plasma treatment resulted in a decrease in water contact angles (WCA) and an increase in the surface free energy (SFE). Water contact angle measurement can be affected by both the chemical composition and the structure of the treated surface. In our case, the presence of the LMWOMs is another factor that had to be taken into account. LMWOMs can be dissolved by polar solvents such as water influencing the measured WCA. For the samples treated at 100 mm/s, surface micro-structure remained unchanged as no difference in the roughness before and after the treatment was observed (
Table 2). Therefore, measured WCAs differences mostly came from the changes in surface chemistry. WCAs of the PP surfaces treated in the Ar and Ar/O
discharge (Ar-
f and ArO-
f) decreased from 99
to 75
. WCAs of the surfaces treated in the Ar/N
plasma (samples ArN-
) were the lowest of the three tested gas feeds with the values of 67
. The decrease in WCAs was likely caused by the addition of nitrogen functionalities as the WCAs decrease with increasing nitrogen content (
Figure 5b,c). The samples treated at 22 mm/s (denoted by an
s) became slightly smoother after the modification (
Table 2). The decrease in roughness was assigned to a combination of the plasma thermal effects and LMWOMs preference to agglomerate in craters. The lowest value of WCA (
)
was measured at the PP surface with the highest concentration of functional groups (sample Ar-
s). Although the surface of the ArN-
s sample contained a higher number of functional groups, both the ArN-
s and ArO-
s samples had the same WCA. Nitrogen functional groups are hydrophilic (although not as much as oxygen functionalities), and a correlation between high nitrogen content and the low WCA was also already shown for higher treatment speed (
Figure 5b,c). Therefore, the higher WCA value must have been caused by the changes in the surface structure not observable by confocal microscopy (
Figure 6a). Kehrer et al. [
17] has recently reported a higher increase in nanoroughness (measured by AFM, the field of view 3 μm) for the PP surface treated in the N
arc-based cold atmospheric plasma jet than for the same jet utilizing air as a process gas. Our Ar/N
PSJ may have a similar effect resulting in the higher final WCA.
Both the components of the SFE have increased for all tested plasma treatment conditions (
Figure 4d). The polar component values varied from the minimum of (
) mJ/m
, the reference, up to (
) mJ/m
, sample Ar-
f. The trend polar SFE values are inverted to the one of WCAs; the lowest WCA corresponded to the highest polar part of the SFE. In the plasma-treated synthetic polymers, the polar part of the SFE is primarily connected to the amount of newly attached polar functional groups (hydroxyl, carboxyl, amines). Thus, the same conclusions used to explain WCA results are also valid for the polar component values. Regardless of the treatment conditions, the value of the dispersive part of the SFE increased from 33 to 37 mJ/m
. The stronger dispersive interaction (induced dipole–induced dipole interaction) between a non-polar liquid and the PP surface was caused by a decrease in molecular orientation in the macrostructure of polypropylene surface (cross-linking, functional group attachment, chain scission, etc.) [
35].
Knowledge of a plasma gas phase can bring insight into the processes happening on the treated PP surface. PSJ operated in an open atmosphere of humid air that intermixed into the active plasma. The optical emission spectra obtained from the region just below the slit exit confirmed the presence of O
2, N
2, H
2O impurities (
Figure 7). In the pure Ar discharge, the most prominent emission lines were from Ar at 690–870 nm, followed by the bands of the second positive system (SPS) of nitrogen molecules, N
(C
–B
) transition. At the UV part of the emission spectrum, N
(C
–B
) bands, (1–0) 315.9 nm and (2–1) 313.6 nm, partially overlapped with the relatively weak OH (A
–X
) bands at 306–310 nm. Among the Ar lines in the VIS part of the spectrum, low-intensity O triplets were present at 777 nm, and 844 nm. The addition of N
into the gas feed lowered emission spectrum intensity by approximately an order of magnitude. The intensity drop was most likely caused by a lower electron density reducing incidence of all the electron excitation processes [
29]. All the lines reported for pure Ar were also observed in the Ar/N
PSJ spectrum. Additionally, the first positive system (FPS) of nitrogen, N
(B
–A
), appeared at 550–900 nm. These emission bands are typical for the orange postdischarge (nitrogen afterglow) [
29], a region rich with nitrogen atoms [
31].
We analyzed the abundance of excited species present in both the Ar and Ar/N plasma by comparing their intensities to the Ar 811.53 nm line. In the Ar PSJ, the relative intensities of O, N(C –B ) and OH (A –X ) transitions were 0.07, 0.7, and 0.07, respectively. The addition of nitrogen into PSJ increased the relative intensity of N(C –B ) and OH (A –X ) bands to 2.7 and 0.3, respectively. The relative intensity of O lines remained unchanged. The slight increase in the relative intensity of OH (A –X ) bands was probably not a result of a larger OH abundance. Presumably, it was caused by OH bands partially overlapping with (1–0) and (2–1) bands of the SPS of N.
Focusing on the plasma treatment, PP can potentially react with O
, O, OH, H, and N. Out of them, PP reactivity with O and OH radicals is significantly higher than with the rest [
26]. The nearly unchanged relative intensity of O and OH emission lines explains the predominant number of oxygen functional groups on all treated PP surfaces (
Table 2). It also explains why the reaction between mid-chain alkyl radicals and N atoms (1) is not prevalent. O and OH radicals are much more reactive, and even their lower numbers can overwhelm the larger amount of N atoms. The nitrogen functional group chemistry is driven by neutral nitrogen atoms. This plasma species cannot be directly observed in OES as the nitrogen excitation energy (14.5 eV) is too high for our type of discharge. Indirectly, for the Ar/N
PSJ, the presence of the afterglow indicates high N atoms concentrations that are nicely reflected by the higher N content on the treated PP surfaces.
3.3. Presence of Low Molecular Weight Oxidized Materials
Most of the plasma-induced functional groups exist at the top of the polymer in the form of low molecular weight oxidized materials (LMWOMs), which are shorter molecular chains created by ablation of a polymer through chain scissions [
17,
38]. In the C 1s high-resolution XPS spectrum, the LMWOMs’ presence can be indicated by the large concentration of ester/carboxyl groups (O=
C–O) terminating the PP chain [
26]. As the LMWOMs are water-soluble, the much easier and straightforward method of identifying their presence is a water-washing test. The comparison of WCAs of water-washed and unwashed samples confirmed LMWOMs’ presence (
Figure 4c). WCAs increased from their initial values (
Figure 4a) to 78–88
, which is still lower than the WCA of pure PP (
)
. Inversely, the values of the polar component of the SFE dropped to 2–5 mJ/m
(
Figure 4f). This suggests that not all functionalized materials exist in the form of LMWOMs. A part of the functional groups must be attached to non-soluble molecular chains of medium or high length that remained on PP surface even after water washing. The dispersive part of the SFE remained unchanged, indicating that the effect of the plasma treatment on the molecular orientation was not limited only to the topmost LMWOM layer but the PP macrostructure situated deeper into the bulk was affected as well.
For all the samples, the water-washing resulted in a major decrease in concentrations of all functional groups (
Figure 9) as the soluble highly functionalized LMWOMs were washed away. Carbon content increased from the initial values (
Table 2) to 90–96 at.% while oxygen content decreased to 4–9 at.%. Nitrogen content was affected by water washing the most. Its concentration either decreased to the XPS detection limit (0.5–1 at.%) or it could not be detected at all (samples with the low initial nitrogen concentration Ar-
f, ArO-
f, and ArO-
s). Out of all oxygen functional groups, the amount of the ester/carboxyl groups (O=
C–O) decreased the most by 60–90%. This was expected, as the O=
C–O groups terminate the PP chain; therefore, most of their population is a part of the short LMWOMs chains. The concentration of the
C–O/
C–N and
C=O/
C=N populations was halved after water washing. Both O 1s environments decreased by similar amounts, by 60–90% depending on the sample. In the N 1s spectrum, the concentration of amine/imine (
N–C/
N=C) was lowered by more than 85% in all samples. It shows that these groups are a part of LMWOMs, most likely as the amide groups at the end of PP chains. The chemical reactions (2)–(4) resulting in the creation of amides were, together with further proofs, discussed in
Section 3.1. The small amount of the
N–C/
N=C remaining at the PP surface was hydrolyzed by water. As a result, the percentage decrease in
N–O concentration was smaller. The roughness after water washing remained the same as the roughness of the plasma-treated surfaces. For some samples, damage to the PP surface inflicted by the plasma was unveiled after the washing. However, the occurrence was random and not connected to certain treatment conditions. Water washing not influencing the roughness suggests that surface craters were filled mostly by medium-length PP chains. Overall, the results confirm the presence of LMWOMs at all treated PP surfaces. The functional group losses were higher for the samples treated at the 22 mm/s with ten passes (denoted with an
s), pointing to a generation of a larger amount of LMWOMs.
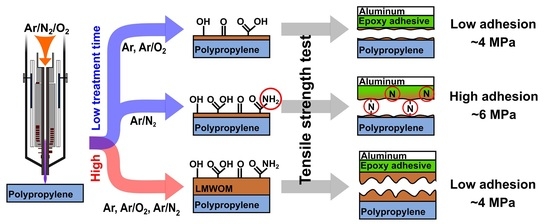
3.4. Adhesion
The present study aimed to provide insight into the mechanism of the adhesive joint between the plasma-treated PP and aluminum. Several adhesives used to create high-strength joints with synthetic polymers are available on the market, but their prices are several times higher than standard epoxy glues. Plasma treatment can allow the usage of low-cost epoxy glues such as the selected epoxy adhesive DP 190 (3M) that has low performance in the joints with synthetic polymers. However, it is necessary to optimize treatment conditions or, even better, to understand the effects that can improve or deteriorate adhesive strength.
The tensile strength of the PP-Al adhesive joint after non-optimized treatment at the 100 mm/s was about three times higher (3.5–4.8 MPa) than for the untreated PP sample (1.4 MPa), as shown in
Table 2. By optimizing the conditions for the Ar/N
gas mixture, we were able to increase the tensile strength of the joints up to 6 MPa (
Figure 5a). Out of the three elements present at the treated PP surfaces (C, O, and N), only N atomic percentages followed any dependency; in
Figure 5c, we can observe how N content increased with higher nitrogen flow rate. In comparison with tensile strength results (
Figure 5a), we can observe a correlation between increased N content and higher tensile strength. The WCAs (
Figure 5b) follow the opposite trend relative to N content and tensile strength. this means that at the selected treatment conditions (Ar/N
gas mixture, 100 mm/s movement speed, two passes), the WCA measurement can be used as a fast method to roughly determine possible adhesion qualities. The correlation between higher nitrogen content and higher tensile strength is connected to the curing process. In the DP 190 adhesive, the epoxy resin is cured by a hardener based on modified amines. The nitrogen groups present on the modified PP surface most likely behaved similarly to the amine groups of the hardener, improving the final adhesion between the adhesive and the PP. Another factor that influenced joint strengths was the applied RF power of the discharge. Higher tensile strengths were observed for samples treated at higher powers, 500 or 600 W (
Figure 5a), meaning both the gas temperature and the number of reactive species were most likely higher. The increase in gas temperature might have promoted a higher degree of melting of the naturally degraded topmost PP layer, improving its bonding to the bulk.
Decreasing the treatment speed from 100 to 22 mm/s and increasing the number of passes from two to ten did not improve the joints strength further even though the number of the functional groups attached to the modified PP surface was significantly higher (
Table 2). For both tested treatment speeds, the XPS analysis of the severed joints revealed the presence of a compact PP layer on the epoxy adhesive (
Figure 10). All joints, therefore, failed cohesively inside PP. Strobel et al. [
19] showed that LMWOMs presence can both positively and negatively impact adhesion. If LMWOMs can be incorporated into the adhesive, LMWOMs act as adhesion enhancers. If not, a weak boundary layer of LMWOMs between the adherent and polymer is created, inhibiting strong adhesion. As the amount of functional groups present on the severed PP surface (5–7 at.%) is similar to their concentration after water washing (4–9 at.%), a weak boundary layer is likely our case. The difference between the tensile strength results for 100 and 22 mm/s movement speed (
Table 2) could be explained by a variation in LMWOM layer thickness. At the lower treatment speed 100 mm/s, LMWOM layer thickness should be low (<10 nm). As a result, a large part of the LMWOMs could be incorporated into the adhesive, leaving the boundary layer thin (at some places perhaps even non-existent) and better connected to both the PP and the adherent. The confocal microscope images (
Figure 6b) support the idea of a thin boundary LMWOMs layer as the PP surface after the tensile strength test maintained the typical globular structure created by the PP granules. The difference in the globules area between the plasma-treated PP before and after the tensile strength test is not a result of experiments but is caused by a variance in the initial PP sheets surfaces. The surface of PP treated at 22 mm/s with ten passes should contain higher amounts LMWOMs, and the boundary layer should be much thicker. For such samples, tensile strength appears to be limited by cohesive forces between LMWOMs as the surface of PP after the tensile strength test is flatter without the globular shapes typical for both the reference and plasma-treated and water-washed samples (
Figure 6a,c might be limited only by the adhesion between the LMWOMs and the PP bulk. The interface between adhesive and LMWOM layer should be further away from PP bulk and play no role. The negative effect of the higher LMWOMs layer thickness is reflected by the number of functional groups on the surface of PP remaining at the adhesive surface. The number was higher for samples treated at 100 mm/s (C ∼ 91 at%) than at 22 mm/s (C ∼ 95 at%).
For RF PSJ, we identified two main parameters influencing adhesion results, N content and amount of LMWOMs. The highest tensile strength (
) MPa was achieved for the PP sample with high N content and a reasonable amount of LMWOMs. This tensile strength value is lower than what we achieved in our previous article [
21], in which we utilized a different type of atmospheric pressure plasma discharge and a gliding arc jet with a side gas inlet. For the movement speed of 100 mm/s and two passes, the maximum tensile strength (
) MPa corresponds to elemental surface composition C:O:N = 71:24:5. The concentration of carboxyl/ethers (O=
C–O) groups, signifying the presence of LMWOMs, was 7.4 at.%. These XPS results are equal to the values achieved for the PP treated by PSJ at 22 mm/s with two passes (
Table 2). The PP treated in the gliding arc jet exhibited higher tensile strength that was twice as high. According to these results, a third important parameter must be influencing adhesion. We identified this parameter as plasma gas temperature. Although the measured rotational temperature of the gliding arc jet (4200 K) does not correspond to its gas temperature, it is still safe to assume that the gliding arc jet is hotter than RF PSJ (
K). During gliding arc jet treatment, the plasma not only creates new functional groups and scissions the PP chains but also slightly melts the topmost polymer layer. The heating of PP results in a higher degree of PP chains cross-linking and, more importantly, fuses LMWOMs and PP bulk. As a result, a weak boundary layer of LMWOMs does not form, and the adhesive joint is much stronger.