Facile and Scalable Synthesis and Self-Assembly of Chitosan Tartaric Sodium
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
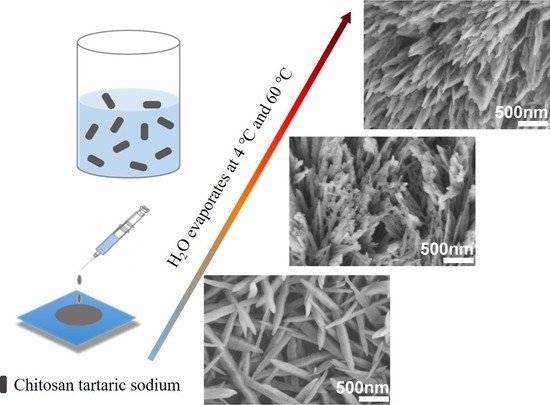
2.2. Sample Preparation
2.3. Instrumentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated chitosans: A multi-talented class of polymers for various applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, H.L.; Wu, D.; Weng, Y.T.; Wang, Z.Y.; Yu, S.H.; Wang, Z. Biomimetic lamellar chitosan scaffold for soft gingival tissue regeneration. Adv. Funct. Mater. 2021, 31, 2105348. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, Y.; Li, P.; Yan, L.; Fan, X.; Wang, Z.; Zhang, W.; Liu, H. Ampholytic Chitosan/Alginate Composite Nanofibrous Membranes with Super Anti-Crude Oil-Fouling Behavior and Multifunctional Oil/Water Separation Properties. ACS Sustain. Chem. Eng. 2019, 7, 15463–15470. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives:A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Cai, Y.; Zhong, Z.; He, C.; Xia, H.; Hu, Q.; Wang, Y.; Ye, Q.; Zhou, J. Homogeneously Synthesized Hydroxybutyl Chitosans in Alkali/Urea Aqueous Solutions as Potential Wound Dressings. ACS Appl. Bio Mater. 2019, 2, 4291–4302. [Google Scholar] [CrossRef]
- Du, Z.; Liu, J.; Zhang, T.; Yu, Y.; Zhang, Y.; Zhai, J.; Huang, H.; Wei, S.; Ding, L.; Liu, B. A study on the preparation of chitosan-tripolyphosphate nanoparticles and its entrapment mechanism for egg white derived peptides. Food Chem. 2019, 286, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Water dispersible pH-responsive chitosan nanogels modified with biocompatible crosslinking-agents. Polymer 2012, 53, 3107–3116. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga, M.D.; Meo, C.D.; Cencetti, C.; Coviello, T.; Matricardi, P.; Alhaique, F. Mechanical and Drug Delivery Properties of a Chitosan–Tartaric Acid Hydrogel Suitable for Biomedical Applications. J. Appl. Polym. Sci. 2011, 123, 842–849. [Google Scholar] [CrossRef]
- Gong, J.; Hu, X.; Wong, K.W.; Zheng, Z.; Yang, L.; Lau, W.M.; Du, R. Chitosan Nanostructures with Controllable Morphology Produced by a Nonaqueous Electrochemical Approach. Adv. Mater. 2008, 20, 2111–2115. [Google Scholar] [CrossRef]
- Duan, B.; Shou, K.; Su, X.; Niu, Y.; Zheng, G.; Huang, Y.; Yu, A.X.; Zhang, Y.; Xia, H.; Zhang, L. Hierarchical Microspheres Constructed from Chitin Nanofibers Penetrated Hydroxyapatite Crystals for Bone Regeneration. Biomacromolecules 2017, 18, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Teramoto, Y. A simple inkjet process to fabricate microstructures of chitinous nanocrystals for cell patterning. Biomacromolecules 2017, 18, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Kurdyla, D.; Hrapovic, S.; Leung, A.C.W.; Régnier, S.; Liu, Y.; Moores, A.; Lam, E. Carboxylated Chitosan Nanocrystals: A Synthetic Route and Application as Superior Support for Gold-Catalyzed Reactions. Biomacromolecules 2020, 21, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Lei, X.; Li, T.; Cheng, Q.; Chang, C.; Hu, L.; Zhang, L. Ultrahigh Tough, Super Clear and Highly Anisotropic Nanofibers-Structured Regenerated Cellulose Films. ACS Nano 2019, 13, 4843–4853. [Google Scholar] [CrossRef]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, S.; Peng, W.; Yin, C.; Wang, W.; Gu, J.; Zhang, W.; Ma, J.; Deng, T.; Feng, C.; et al. Bioinspired Fabrication of Hierarchically Structured, pH-Tunable Photonic Crystals with Unique Transition. ACS Nano 2013, 7, 4911–4918. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Q.; Shi, L.; Zhang, X.; Zhang, K.Q. Bio-inspired sensors based on photonic structures of Morpho butterfly wings: A review. J. Mater. Chem. C 2016, 4, 1752–1763. [Google Scholar] [CrossRef]
- Liu, C.; Xu, G.; Du, J.; Sun, J.; Wan, X.; Liu, X.; Su, J.; Liang, J.; Zheng, G.; Xie, L.; et al. Mineralization of Nacre-like Structures Mediated by Extrapallial Fluid on Pearl Nucleus. Cryst. Growth Des. 2017, 18, 32–36. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Peres, B.U.; Carvalho, R.M.; MacLachlan, M.J. Photonic Hydrogels from Chiral Nematic Mesoporous Chitosan Nanofi bril Assemblies. Adv. Funct. Mater. 2016, 26, 2875–2881. [Google Scholar] [CrossRef]
- Huang, G.; Yin, Y.; Pan, Z.; Chen, M.; Zhang, L.; Liu, Y.; Zhang, Y.; Gao, J. Fabrication of 3D Photonic Crystals from Chitosan That Are Responsive to Organic Solvents. Biomacromolecules 2014, 15, 4396–4402. [Google Scholar] [CrossRef]
- Retamal, M.J.; Corrales, T.P.; Cisternas, M.; Moraga, N.; Diaz, D.; Catalan, R.; Seifert, B.; Huber, P.; Volkmann, U.G. Surface morphology of vapor-deposited chitosan: Evidence of solid-state dewetting during the formation of biopolymer films. Biomacromolecules 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Duan, J.; Guo, J.; Wu, S.; Lu, A.; Zhang, L. High-Strength Films Consisted of Oriented Chitosan Nanofibers for Guiding Cell Growth. Biomacromolecules 2017, 18, 3904–3912. [Google Scholar] [CrossRef]
- Chen, J.; Qin, S.; Wu, X.; Chu, P.K. Morphology and Pattern Control of Diphenylalanine Self-Assembly via Evaporative Dewetting. ACS Nano 2015, 10, 832–838. [Google Scholar] [CrossRef]
- Qin, J.; Luo, T.; Kiick, K.L. Self-assembly of stable nanoscale platelets from designed elastin-like peptide—Collagen-like peptide bioconjugates. Biomacromolecules 2019, 20, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Demarger-Andre, S.; Domard, A. Chitosan carboxylic acid salts in solution and in the solid state. Carbohydr. Polym. 1994, 23, 211–219. [Google Scholar] [CrossRef]
- Huang, J.; Xie, H.H.; Hu, S.; Gong, J.Y.; Jiang, C.J.; Xie, T.; Ge, Q.; Wu, Y.; Cui, Y.; Liu, S.W.; et al. Preparation, Characterization and Biochemical Activities of N-(2-Carboxyethyl)chitosan from Squid Pens. J. Agric. Food Chem. 2015, 63, 2464–2471. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-healing Hydrogels Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Akao, H.; Yang, J.; Shimojoh, M. Nonnatural Branched Polysaccharides: Synthesis and Properties of Chitin and Chitosan Having Disaccharide Maltose Branches. Biomacromolecules 2003, 4, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Phongying, S.; Aiba, S.I.; Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, R.; Duan, B.; Liu, M.; Lu, A.; Zhang, L. Recyclable Universal Solvents for Chitin to Chitosan with Various Degrees of Acetylation and Construction of Robust Hydrogels. ACS Sustain. Chem. Eng. 2017, 5, 2725–2733. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated Chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Zhou, J.; Man, X.; Jiang, Y.; Doi, M. Structure Formation in Soft-Matter Solutions Induced by Solvent Evaporation. Adv. Mater. 2017, 29, 1703769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohani, D.; Basavaraj, M.G.; Satapathy, D.K.; Sarkar, S. Coupled effect of concentration, particle size and substrate morphology on the formation of coffee rings. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 589, 124387. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Peng, R.; Bian, S.; Han, W.; Xiao, B.; Peng, X. Facile and Scalable Synthesis and Self-Assembly of Chitosan Tartaric Sodium. Polymers 2022, 14, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010069
Wei S, Peng R, Bian S, Han W, Xiao B, Peng X. Facile and Scalable Synthesis and Self-Assembly of Chitosan Tartaric Sodium. Polymers. 2022; 14(1):69. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010069
Chicago/Turabian StyleWei, Sixuan, Rujie Peng, Shilong Bian, Wei Han, Biao Xiao, and Xianghong Peng. 2022. "Facile and Scalable Synthesis and Self-Assembly of Chitosan Tartaric Sodium" Polymers 14, no. 1: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010069