Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
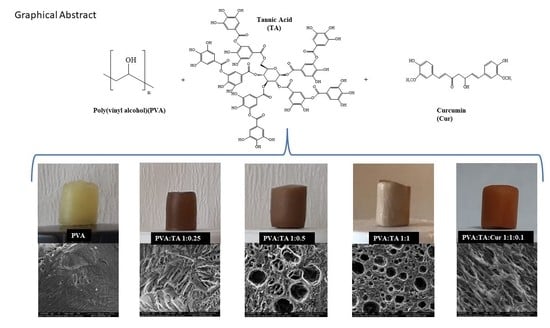
2.2. Synthesis of PVA Cryogel, PVA:TA, and PVA:TA:Cur Cryogel Composites
2.3. Characterization of PVA:TA and PVA:TA:Cur Cryogel Composites
2.4. TA and Cur Release Profiles from PVA:TA and PVA:TA:Cur Cryogel Composites
2.5. Hemocompatibility Testing for PVA Cryogel, PVA:TA, and PVA:TA:Cur Cryogel Composites
2.6. Antioxidant Features of PVA:TA and PVA:TA:Cur Cryogel Composites
2.6.1. Total Phenol Content (TPC) Assay
2.6.2. Trolox Equivalent Antioxidant (TEAC) Assay
2.7. Determination of Antimicrobial Effects of PVA Cryogel, PVA:TA, and PVA:TA:Cur Cryogel Composites
Macro Dilution Method
2.8. Enzymatic Assay
3. Results
3.1. Synthesis and Characterization of PVA Cryogel, PVA:TA, and PVA:TA:Cur Cryogel Composites
3.2. TA and Cur Release from Cryogel/Composite Matrices
3.3. Hemocompatibility Test
3.4. Antioxidant Features
3.5. Antimicrobial Activity
3.6. Enzymatic Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozinsky, V.I. A Brief History of Polymeric Cryogels. In Polymeric Cryogels; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–48. [Google Scholar]
- Zhang, Q.; Hu, X.M.; Wu, M.Y.; Wang, M.M.; Zhao, Y.Y.; Li, T.T. Synthesis and performance characterization of poly(vinyl alcohol)-xanthan gum composite hydrogel. React. Funct. Polym. 2019, 136, 34–43. [Google Scholar] [CrossRef]
- Harpaz, D.; Axelrod, T.; Yitian, A.; Eltzov, E.; Marks, R.; Tok, A. Dissolvable Polyvinyl-Alcohol Film, a Time-Barrier to Modulate Sample Flow in a 3D-Printed Holder for Capillary Flow Paper Diagnostics. Materials 2019, 12, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, A.K.; Vishwakarma, A.; Bajpai, J. Synthesis and characterization of amoxicillin loaded poly (vinyl alcohol)-g-poly (acrylamide) (PVA-g-PAM) hydrogels and study of swelling triggered release of antibiotic drug. Polym. Bull. 2019, 76, 3269–3295. [Google Scholar] [CrossRef]
- Caló, E.; Barros, J.; Ballamy, L.; Khutoryanskiy, V.V. Poly(vinyl alcohol)–Gantrez® AN cryogels for wound care applications. RSC Adv. 2016, 6, 105487–105494. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.H. Polyvinyl alcohol/tannic acid hydrogel prepared by a freeze-thawing process for wound dressing applications. Polym. Bull. 2017, 74, 2861–2872. [Google Scholar] [CrossRef]
- Sundaramahalingam, K.; Muthuvinayagam, M.; Nallamuthu, N.; Vanitha, D.; Vahini, M. Investigations on lithium acetate-doped PVA/PVP solid polymer blend electrolytes. Polym. Bull. 2019, 76, 5577–5602. [Google Scholar] [CrossRef]
- Piluso, P.; Sudre, G.; Boisson-Da Cruz, F.; Bounor-Legaré, V.; Espuche, E. Impact of 10-undecenal PVA acetalization on the macromolecular organization and the viscosity of aqueous solutions. Surface and bulk properties of the modified PVA films. Eur. Polym. J. 2018, 108, 412–419. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, X.; Pan, M.; Yuan, J.; Jia, Z.; Zhu, L. A Robust, Tough and Multifunctional Polyurethane/Tannic Acid Hydrogel Fabricated by Physical-Chemical Dual Crosslinking. Polymers 2020, 12, 239. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Cao, K.; Li, W.; Li, X.; McClements, D.J.; Hu, K. Tannic acid-fortified zein-pectin nanoparticles: Stability, properties, antioxidant activity, and in vitro digestion. Food Res. Int. 2021, 145, 110425. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C. P(TA) macro-, micro-, nanoparticle-embedded super porous p(HEMA) cryogels as wound dressing material. Mater. Sci. Eng. C 2017, 70, 317–326. [Google Scholar] [CrossRef]
- Luo, L.J.; Lai, J.Y.; Chou, S.F.; Hsueh, Y.J.; Ma, D.H.K. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, C.; Chen, J.; Wang, Y.; Liang, R.; Zou, L.; McClements, D.J.; Liu, W. Enhancement of beta-carotene stability by encapsulation in high internal phase emulsions stabilized by modified starch and tannic acid. Food Hydrocoll. 2020, 109, 106083. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Wekwejt, M.; Nadolna, K.; Owczarek, A.; Mazur, O.; Pałubicka, A. The mechanical properties and bactericidal degradation effectiveness of tannic acid-based thin films for wound care. J. Mech. Behav. Biomed. Mater. 2020, 110, 103916. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Lin, M.; Zhang, C.; Shi, J.; Zhang, S.; Chen, Q.; Hu, Y.; Zhang, M.; Zhang, J.; Gao, F. Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chem. 2020, 330, 127241. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Niu, B.; Luo, Q.; Zhang, Y.; Quan, G.; Pan, X.; Wu, C. Cyclodextrin-based metal-organic frameworks for pulmonary delivery of curcumin with improved solubility and fine aerodynamic performance. Int. J. Pharm. 2020, 588, 119777. [Google Scholar] [CrossRef]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Zhu, M.; Qiu, F.; Li, W.; Wang, M.; Guo, Y.; Xi, X.; Du, B. Curcumin may be a potential adjuvant treatment drug for colon cancer by targeting CD44. Int. Immunopharmacol. 2020, 88, 106991. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.; Wang, K.; Zhu, X.; Chen, D.; Zhuang, H.; Yao, L.; Song, S.; Wang, H.; Sun, M. Emulsion-based delivery systems for curcumin: Encapsulation and interaction mechanism between debranched starch and curcumin. Int. J. Biol. Macromol. 2020, 161, 746–754. [Google Scholar] [CrossRef]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhao, Y.; Matthews, K.; Gao, J.; Hao, J.; Wang, S.; Han, J.; Jia, Y. Antibacterial activity against Staphylococcus aureus of curcumin-loaded chitosan spray coupled with photodynamic treatment. LWT 2020, 134, 110073. [Google Scholar] [CrossRef]
- Thapa, R.K.; Cazzador, F.; Grønlien, K.G.; Tønnesen, H.H. Effect of curcumin and cosolvents on the micellization of Pluronic F127 in aqueous solution. Colloids Surf. B Biointerfaces 2020, 195, 111250. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Rajasekar, A.; Devasena, T.; Suresh, S.; Senthil, B.; Sivaramakrishnan, R.; Pugazhendhi, A. Curcumin nanospheres and nanorods: Synthesis, characterization and anticancer activity. Process Biochem. 2021, 112, 248–253. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; He, Y.; Liu, C.; Ni, F.; Luo, X.; Shi, J.; Song, Y.; Li, T.; Huang, M.; Shen, Q.; et al. Encapsulation of curcumin in ZEIN-HTCC complexes: Physicochemical characterization, in vitro sustained release behavior and encapsulation mechanism. LWT 2022, 155, 112909. [Google Scholar] [CrossRef]
- Zoughaib, M.; Luong, D.; Garifullin, R.; Gatina, D.Z.; Fedosimova, S.V.; Abdullin, T.I. Enhanced angiogenic effects of RGD, GHK peptides and copper (II) compositions in synthetic cryogel ECM model. Mater. Sci. Eng. C 2021, 120, 111660. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Guo, B.; Yin, Z.; Zhu, D.; Han, Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chem. Eng. J. 2021, 403, 126329. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lou, X.; Xu, R.; Dai, C.; Xu, N.; Yan, Q.; Chen, M.; Sun, X.; Zhu, L.; Yun, J.; et al. Hydrophobic cryogels prepared via cryo-polymerization as oil carriers for biosynthesis of sophorolipids. Biochem. Eng. J. 2020, 161, 107677. [Google Scholar] [CrossRef]
- Lozinsky, V. Cryostructuring of Polymeric Systems. 50.† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Bölgen, N.; Demir, D.; Yalçın, M.S.; Özdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Andrabi, S.M.; Singh, A.; Majumder, S.; Kumar, A. Designing cryogels through cryostructuring of polymeric matrices for biomedical applications. Eur. Polym. J. 2021, 144, 110234. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Morariu, S.; Bercea, M.; Săcărescu, L. Viscoelastic and structural properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels. RSC Adv. 2016, 6, 39718–39727. [Google Scholar] [CrossRef]
- Rakesh, G.; Deshpande, A.P. Rheology of crosslinking poly vinyl alcohol systems during film formation and gelation. Rheol. Acta 2010, 49, 1029–1039. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J. Preparation and characterization of poly(vinyl alcohol) cryogel-silver nanocomposites and evaluation of blood compatibility, cytotoxicity, and antimicrobial behaviors. Polym. Compos. 2015, 36, 1983–1997. [Google Scholar] [CrossRef]
- Ari, B.; Yetiskin, B.; Okay, O.; Sahiner, N. Preparation of dextran cryogels for separation processes of binary dye and pesticide mixtures from aqueous solutions. Polym. Eng. Sci. 2020, 60, 1890–1901. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine particles as nontoxic, blood compatible, antioxidant and drug delivery materials. Colloids Surf. B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, Y.; Liu, X.; Zhang, H. Ecofriendly construction of enzyme reactor based on three-dimensional porous cryogel composites. Chem. Eng. J. 2019, 361, 286–293. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. International Journal of Biological Macromolecules Preparation and characterization of polylactic acid / fenugreek essential oil / curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2021, 194, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, İ.; Tuna, S. Biowaste orange peel incorporated chitosan/polyvinyl alcohol composite films for food packaging applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-hady, E.E.; Mohamed, H.F.M.; Abdel-hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Nam, S.; Easson, M.W.; Condon, B.D.; Hillyer, M.B.; Sun, L.; Xia, Z.; Nagarajan, R. A reinforced thermal barrier coat of a Na–tannic acid complex from the view of thermal kinetics. RSC Adv. 2019, 9, 10914–10926. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Wilson, D.; Nash, P.; Buttar, H.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Mandel, S.A.; Avramovich-Tirosh, Y.; Reznichenko, L.; Zheng, H.; Weinreb, O.; Amit, T.; Youdim, M.B.H. Multifunctional Activities of Green Tea Catechins in Neuroprotection. Neurosignals 2005, 14, 46–60. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting- In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar] [CrossRef]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Sahiner, M. Hydrolytic nondegradable bioactive rosmarinic acid particles. Polym. Adv. Technol. 2021, 32, 4891–4901. [Google Scholar] [CrossRef]
- Deng, L.; Qi, Y.; Liu, Z.; Xi, Y.; Xue, W. Effect of tannic acid on blood components and functions. Colloids Surf. B Biointerfaces 2019, 184, 110505. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Qiao, Z.; Lv, X.; He, S.; Bai, S.; Liu, X.; Hou, L.; He, J.; Tong, D.; Ruan, R.; Zhang, J.; et al. A mussel-inspired supramolecular hydrogel with robust tissue anchor for rapid hemostasis of arterial and visceral bleedings. Bioact. Mater. 2021, 6, 2829–2840. [Google Scholar] [CrossRef]
- Pan, W.; Qi, X.; Xiang, Y.; You, S.; Cai, E.; Gao, T.; Tong, X.; Hu, R.; Shen, J.; Deng, H. Facile formation of injectable quaternized chitosan/tannic acid hydrogels with antibacterial and ROS scavenging capabilities for diabetic wound healing. Int. J. Biol. Macromol. 2022, 195, 190–197. [Google Scholar] [CrossRef]







| Materials | S% | M% | P% | Vp (mL/g) |
|---|---|---|---|---|
| PVA | 169 ± 6 | 63 ± 2 | 8 ± 4 | 0.10 ± 0.05 |
| PVA:TA 1:0.1 | 198 ± 17 | 66 ± 2 | 10 ± 3 | 0.13 ± 0.06 |
| PVA:TA 1:0.25 | 239 ± 23 | 71 ± 2 | 10 ± 1 | 0.19 ± 0.07 |
| PVA:TA 1:0.5 | 239 ± 91 | 71 ± 9 | 17 ± 1 | 0.27 ± 0.17 |
| PVA:TA 1:1 | 258 ± 62 | 72 ± 6 | 43 ± 5 | 0.69 ± 0.32 |
| PVA:TA:Cur 1:1:0.1 | 229 ± 31 | 69 ± 3 | 38 ± 5 | 0.66 ± 0.19 |
| Release Conditions | Antioxidant Materials | TPC (µg/mL) | TEAC (µmole TE/g) |
|---|---|---|---|
| pH 7.4 | PVA:TA 1:0.1 | 5.14 ± 0.26 | 0.06 ± 0.01 |
| PVA:TA 1:0.25 | 18.45 ± 0.13 | 0.11 ± 0.01 | |
| PVA:TA 1:0.5 | 54.62 ± 1.33 | 0.17 ± 0.05 | |
| PVA:TA 1:1 | 65.28 ± 0.11 | 0.75 ± 0.01 | |
| EtOH | PVA:TA:Cur 1:1:0.1 | 235.41 ± 4.00 | 2.01 ± 0.22 |
| EtOH:Wat | PVA:TA:Cur 1:1:0.1 | 292.71 ± 11.50 | 2.10 ± 0.24 |
| Organisms | E. coli | S. aureus | ||
|---|---|---|---|---|
| Antimicrobial Materials | MIC (mg/mL) | MBC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) |
| PVA | N.D. | N.D. | N.D. | N.D. |
| PVA:TA 1:0.1 | N.D. | N.D. | N.D. | N.D. |
| PVA:TA 1:0.25 | N.D. | N.D. | N.D. | N.D. |
| PVA:TA 1:0.5 | 10 | 20 | 10 | 20 |
| PVA:TA 1:1 | 10 | 20 | 5 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ari, B.; Sahiner, M.; Demirci, S.; Sahiner, N. Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material. Polymers 2022, 14, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010070
Ari B, Sahiner M, Demirci S, Sahiner N. Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material. Polymers. 2022; 14(1):70. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010070
Chicago/Turabian StyleAri, Betul, Mehtap Sahiner, Sahin Demirci, and Nurettin Sahiner. 2022. "Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material" Polymers 14, no. 1: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010070