Designing Phenyl Porous Organic Polymers with High-Efficiency Tetracycline Adsorption Capacity and Wide pH Adaptability
Abstract
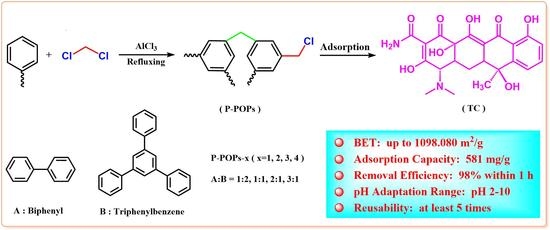
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Typical Procedure for the Synthesis of P-POPs
2.3. Characterization of Samples
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Structural Analysis and Characterization of Material
3.2. Effect of Adsorbent
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. The Effect of pH
3.6. Reused of the Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H.; Tian, Y.; Zhu, G. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J. 2020, 382, 122893. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Ifebajo, A.O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef]
- Liang, Y.; Pei, M.; Wang, D.; Cao, S.; Xiao, X.; Sun, B. Improvement of soil ecosystem multifunctionality by dissipating manure-induced antibiotics and resistance genes. Environ. Sci. Technol. 2017, 51, 4988–4998. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.; Bandoch, G.F.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.S. Disrupting effects of antibiotic sulfathiazole on developmental process during sensitive life-cycle stage of Chironomus riparius. Chemosphere 2018, 190, 25–34. [Google Scholar] [CrossRef]
- Wang, T.; Pan, X.; Ben, W.; Wang, J.; Hou, P.; Qiang, Z. Adsorptive removal of antibiotics from water using magnetic ion exchange resin. J. Environ. Sci. 2017, 52, 111–117. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Wang, Z.; Wang, H.; Deng, C.; Li, K. Effective degradation of aqueous tetracycline using a nano-TiO2/carbon electrocatalytic membrane. Materials 2016, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liang, Y.; Zou, D.; Yuan, X.; Xiao, Z.; Deng, Y.; Zhou, Y.; Jiang, L.; Qin, P. Enhanced heterogeneous activation of persulfate by NixCo3–xO4 for oxidative degradation of tetracycline and bisphenol A. J. Environ. Chem. Eng. 2020, 8, 104451. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Prominent co-catalytic effect of CoP nanoparticles anchored on high-crystalline g-C3N4 nanosheets for enhanced visible-light photocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2020, 395, 125118. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Kareem, S.L. Adsorption of tetracycline fom wastewater by using Pistachio shell coated with ZnO nanoparticles: Equilibrium, kinetic and isotherm studies. Alex. Eng. J. 2019, 58, 917–928. [Google Scholar] [CrossRef]
- Chen, G.X.; Li, H.F.; Wang, D.D.; Li, S.Q.; Fan, X.B.; Zhang, J.M. Adsorption of toxic gas molecules on pristine and transition metal doped hexagonal GaN monolayer: A first-principles study. Vacuum 2019, 165, 35–45. [Google Scholar] [CrossRef]
- Chen, G.X.; Wang, R.X.; Wang, D.D.; Li, H.X.; Liu, S.; Zhang, J.M. First-principles study of CO and NO adsorption on pristine and transition metal doped blue phosphorene. Vacuum 2020, 179, 109503. [Google Scholar] [CrossRef]
- Debnath, B.; Majumdar, M.; Bhowmik, M.; Bhowmik, K.L.; Debnath, A.; Roy, D.N. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J. Environ. Manag. 2020, 261, 110235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Zhou, J.; Ni, B. Anaerobic membrane bioreactors for antibiotic wastewater treatment: Performance and membrane fouling issues. Bioresour. Technol. 2018, 267, 714–724. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Yao, B.; Zou, D. Removal of chlortetracycline by nano-micro-electrolysis materials: Application and mechanism. Chemosphere 2020, 238, 124543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid. Interf. Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.L.; Li, C.E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total Environ. 2019, 651, 580–590. [Google Scholar] [CrossRef]
- Yang, J.; Dou, Y.; Yang, H.; Wang, D. A novel porous carbon derived from CO2 for high-efficient tetracycline adsorption: Behavior and mechanism. Appl. Surf. Sci. 2021, 538, 148110. [Google Scholar] [CrossRef]
- Chang, P.H.; Li, Z.; Jean, J.S.; Jiang, W.T.; Wang, C.J.; Lin, K.H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 2012, 67, 158–163. [Google Scholar] [CrossRef]
- Miao, J.; Wang, F.; Chen, Y.; Zhu, Y.; Zhou, Y.; Zhang, S. The adsorption performance of tetracyclines on magnetic graphene oxide: A novel antibiotics absorbent. Appl. Surf. Sci. 2019, 475, 549–558. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, F.; Guo, H. Adsorption behavior of tetracycline from aqueous solution on ferroferric oxide nanoparticles assisted powdered activated carbon. Chem. Eng. J. 2020, 384, 123290. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Chaudhary, A.A.; Naushad, M.; Alshehri, S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019, 134, 180–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Liu, X.; Sheng, N.; Deng, F.; Meng, X.; Xiao, F.S. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: Synergistic effect of high ligand concentration and flexible framework. J. Am. Chem. Soc. 2015, 137, 5204–5209. [Google Scholar] [CrossRef]
- Ji, G.; Yang, Z.; Yu, X.; Zhao, Y.; Zhang, F.; Liu, Z. Photosensitive Hyper-Cross-Linked Polymers Derived from Three-Dimensional Ringlike Arenes: Promising Catalysts for Singlet-Oxygen Generation. ACS. Sustain. Chem. Eng. 2020, 8, 16320–16326. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Shi, C.; Guo, F.; He, C.; Cao, Z.; Hu, J.; Cui, C.; Liu, H. Induced-fit adsorption of diol-based porous organic polymers for tetracycline removal. Chemosphere 2018, 212, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Q.; Liu, Y.; Meng, X.; Ye, Y.; Liu, X.; Song, X.; Liang, Z. Multifunctional conjugated microporous polymers with pyridine unit for efficient iodine sequestration, exceptional tetracycline sensing and removal. J. Hazard. Mater. 2020, 387, 121949. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Choi, T.R.; Gurav, R.; Bhatia, S.K.; Park, Y.L.; Kim, H.J.; Kan, E.; Yang, Y.H. Adsorption behavior of tetracycline onto Spirulina sp.(microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef] [PubMed]
- Rozyyev, V.; Thirion, D.; Ullah, R.; Lee, J.; Jung, M.; Oh, H.; Atilhan, M.; Yavuz, C.T. High-capacity methane storage in flexible alkane-linked porous aromatic network polymers. Nat. Energy 2019, 4, 604–611. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, W.; Zhu, Z.; Chen, G.; Ma, L.; Ye, S.; Niu, Q. A covalent triazine-based framework from tetraphenylthiophene and 2, 4, 6-trichloro-1, 3, 5-triazine motifs for sensing o-nitrophenol and effective I2 uptake. Polym. Chem. 2018, 9, 777–784. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K. Interaction of tetracycline with aluminum and iron hydrous oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef]










| Sample | SBET (m2/g) | Vmax (cm3/g) | Wmed/nm |
|---|---|---|---|
| P-POPs-1 | 571 | 0.305 | 0.678 |
| P-POPs-2 | 580 | 0.311 | 0.637 |
| P-POPs-3 | 1098 | 0.579 | 0.698 |
| P-POPs-4 | 908 | 0.481 | 0.685 |
| qexp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (1/min) | qmax (mg/g) | R2 | k2 (g/(mg·min)) | qmax (mg/g) | R2 | |
| 196 | 0.01512 | 9.0 | 0.639 | 0.0014 | 198.4 | 0.999 |
| Langmuir Model | Freundlich Model | Sips Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | kL (L/mg) | R2 | kF (mg/g) | n | R2 | qm (mg/g) | b (L/mg) | n | R2 |
| 568 | 0.5297 | 0.9829 | 221.3034 | 4.6906 | 0.9078 | 581 | 0.5833 | 1.1480 | 0.9850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Liu, J.; Bai, X.; Xing, Z.; Gao, Y. Designing Phenyl Porous Organic Polymers with High-Efficiency Tetracycline Adsorption Capacity and Wide pH Adaptability. Polymers 2022, 14, 203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010203
Nie W, Liu J, Bai X, Xing Z, Gao Y. Designing Phenyl Porous Organic Polymers with High-Efficiency Tetracycline Adsorption Capacity and Wide pH Adaptability. Polymers. 2022; 14(1):203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010203
Chicago/Turabian StyleNie, Wenjie, Jiao Liu, Xue Bai, Zefeng Xing, and Ying Gao. 2022. "Designing Phenyl Porous Organic Polymers with High-Efficiency Tetracycline Adsorption Capacity and Wide pH Adaptability" Polymers 14, no. 1: 203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14010203