The Influence of Synthesis Method on Characteristics of Buffer and Organic Solutions of Thermo- and pH-Responsive Poly(N-[3-(diethylamino)propyl]methacrylamide)s
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monomer Synthesis
2.2. Synthesis of the Polymer of N-[3-(diethylamino)propyl]methacrylamide by Free Radical Polymerization
2.3. Synthesis of the Polymer of N-[3-(diethylamino)propyl]methacrylamide by RAFT Polymerization
3. Instrumentations
3.1. Determination of Molar Mass and Hydrodynamic Characteristics of Poly(N-[3-(diethylamino)propyl]methacrylamide)s
3.2. Investigation of Self-Assembly of Poly(N-[3-(diethylamino)propyl]methacrylamide)s in Buffer Solutions
4. Results and Discussion
4.1. Polymer Synthesis and Characterization
4.2. Characteristics of Poly(N-[3-(diethylamino)propyl]methacrylamide)s in Buffer Solutions at Room Temperatures
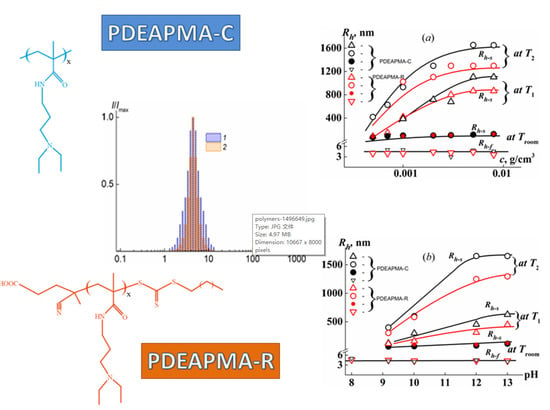
4.3. Temperature Dependences of the Characteristics of Poly(N-[3-(diethylamino)propyl]meth Acrylamide)s in Buffer Solutions
4.4. Concentration and pH Dependences of the Characteristics of Poly(N-[3-(diethylamino)propyl]meth Acrylamide)s in Buffer Solutions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef] [Green Version]
- Simonova, M.A.; Kamorin, D.M.; Kazantsev, O.A.; Nepomnyashaya, M.I.; Filippov, A.P. Conformation, Self-Organization and Thermoresponsibility of Polymethacrylate Molecular Brushes with Oligo(ethylene glycol)-block-oligo(propylene glycol) Side Chains. Polymers 2021, 13, 2715. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Elmas, B.; Tuncel, M.; Tuncel, A. A new, highly stable cationic-thermosensitive microgel: Uniform isopropylacrylamide-dimethylaminopropylmethacrylamide copolymer particles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 247–253. [Google Scholar] [CrossRef]
- Jean, B.; Bokias, G.; Lee, L.-T.; Iliopoulos, I.; Cabane, B. Microphase separation of cationic poly(N-isopropylacrylamide) copolymers in water: Effect of the migration of charges. Colloid Polym. Sci. 2002, 280, 908–914. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A. pH-responsive poly(DMAPMA-co-HEMA)-based hydrogels for prolonged release of 5-fluorouracil. J. Appl. Polym. Sci. 2012, 126, 98–107. [Google Scholar] [CrossRef]
- Çaykara, T.; Birlik, G. Synthesis and network parameters of hydrophobic poly(N-[3-(dimethylaminopropyl)]methacrylamide-co-lauryl acrylate) hydrogels. J. Appl. Polym. Sci. 2006, 101, 4159–4166. [Google Scholar] [CrossRef]
- Das, A.; Mehndiratta, M.; Chattopadhyay, P.; Ray, A.R. Prolonged zero-order BSA release from pH-sensitive hydrogels of poly(AAc-co-DMAPMA) having rich nano through micro scale morphology. J. Appl. Polym. Sci. 2010, 115, 393–403. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.; Ray, A.R. Unveiling the self-assembly behavior of copolymers of AAc and DMAPMA in situ to form smart hydrogels displaying nanogels-within-macrogel hierarchical morphology. Polymer 2011, 52, 3800–3810. [Google Scholar] [CrossRef]
- Demirel, G.B.; Çaykara, T. Reentrant phase transition and fast responsive behaviors of poly{N-[3-(dimethylaminopropyl)]methacrylamide} hydrogels prepared in poly(ethylene glycol) solutions. J. Appl. Polym. Sci. 2009, 113, 547–552. [Google Scholar] [CrossRef]
- Schmitz, S.; Ritter, H. Access to Poly{N-[3-(dimethylamino)propyl](meth)acrylamide} via Microwave-Assisted Synthesis and Control of LCST-Behavior in Water. Macromol. Rapid Comm. 2007, 28, 2080–2083. [Google Scholar] [CrossRef]
- Song, Z.; Wang, K.; Gao, C.; Wang, S.; Zhang, W. A New Thermo-, pH-, and CO2-Responsive Homopolymer of Poly[N-[2-(diethylamino)ethyl]acrylamide]: Is the Diethylamino Group Underestimated. Macromolecules 2016, 49, 162–171. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.; Liu, C.; Zhang, W. RAFT synthesis of triply responsive poly[N-[2-(dialkylamino)ethyl]acrylamide]s and their N-substitute determined response. Polym. Chem. 2016, 7, 3423–3433. [Google Scholar] [CrossRef]
- Simonova, M.A.; Zakharova, N.V.; Khayrullin, A.R.; Filippov, A.P. Behavior of double stimuli responsive behavior of N-(3-(diethylamino)propyl)-N-methylacrylamide and N,N–diethylacrylamide in aqueous solutions. Int. J. Polym. Anal. Charact. 2018, 23, 236–243. [Google Scholar] [CrossRef]
- Simonova, M.A.; Khayrullin, A.R.; Tyurina, V.O.; Filippov, A.P.; Sadikov, A.Y.; Kamorin, D.M.; Kamorina, S.I. Self-Organization Processes in Poly(N-[2-(diethylamino)ethyl]acryl amide) Buffer Solutions with Change in Concentration and pH of a Medium. Polym. Sci. Ser. A 2020, 62, 24–31. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Wischerhoff, E. Modulating the solubility of zwitterionic poly((3-methacrylamidopropyl)ammonioalkane sulfonate)s in water and aqueous salt solutions via the spacer group separating the cationic and the anionic moieties. Polym. Chem. 2016, 7, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Yuk, S.H.; Cho, S.H.; Lee, S.H. pH/Temperature-Responsive Polymer Composed of Poly((N,N-dimethylamino)ethyl methacrylate-co-ethylacrylamide). Macromolecules 1997, 30, 6856–6859. [Google Scholar] [CrossRef]
- Danilovtseva, E.N.; Aseyev, V.O.; Beloserova, O.Y.; Zelinskiy, S.N.; Annenkov, V.V. Bioinspired thermo- and pH-responsive polymeric amines: Multimolecular aggregates in aqueous media and matrices for silica/polymer nanocomposites. J. Colloid Interface Sci. 2015, 446, 1–10. [Google Scholar] [CrossRef]
- Yuan, J.; Peng, J.; Li, J.; Ju, X.; Zhai, M. Synthesis of poly(dimethylaminoethyl methacrylate) with high cloud point by RAFT polymerization under γ-irradiation. Radiat. Phys. Chem. 2015, 108, 95–101. [Google Scholar] [CrossRef]
- Ros, S.; Burke, N.A.D.; Stover, H.D.H. Synthesis and Properties of Charge-Shifting Polycations: Poly[3-aminopropylmethacrylamide-co-2-(dimethylamino)ethyl acrylate]. Macromolecules 2015, 48, 8958–8970. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.W.; Mc Cormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2016, 8, 177–219. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Oguri, A.; Minoda, M. Synthesis of Well-Defined Alternating Copolymer Composed of Ethylmaleimide and Hydroxy-Functionalized Vinyl Ether by RAFT Polymerization and Their Thermoresponsive Properties. Polymers 2020, 12, 2255. [Google Scholar] [CrossRef]
- Mori, H.; Iwaya, H.; Endo, T. Controlled synthesis of thermoresponsive polymer via RAFT polymerization of an acrylamide containing L-proline moiety. React. Funct. Polym. 2007, 67, 916–927. [Google Scholar] [CrossRef]
- Paricaud, P.; Galindo, A.; Jackson, G. Examining the effect of chain length polydispersity on the phase behavior of polymer solutions with the statistical associating fluid theory (Wertheim TPT1) using discrete and continuous distributions. J. Chem. Phys. 2007, 127, 154906. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Khatir, N.M. Investigation of acyclovir-loaded, acrylamide-based hydrogels for potential use as vaginal ring. Mat. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Physical and chemical/characterisation of acrylamide-based hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solutions; Elsevier: Amsterdam, The Netherlands, 1987; p. 334. [Google Scholar]
- Schartl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin, Germany, 2007; p. 175. [Google Scholar]
- Geetha, B.; Mandal, A.B.; Tirumalachari, R. Synthesis, characterization, and micelle formation in an aqueous solution of methoxypolyethylene glycol macromonomer, homopolymer, and graft copolymer. Macromolecules 1993, 26, 4083–4088. [Google Scholar] [CrossRef]
- Sivokhin, A.P.; Orekhov, D.V.; Kazantsev, O.A.; Gubanova, O.V.; Kamorin, D.M.; Zarubina, I.S.; Bolshakova, E.A.; Zaitsev, S.D. Amphiphilic thermoresponsive copolymer bottlebrushes: Synthesis, characterization, and study of their self-assembly into flower-like micelles. Polym. J. 2021, 53, 655–665. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Eskin, V.E.; Frenkel, S.Y. Structure of Macromolecules in Solution; Izd. “Nauka”: Moscow, Russia, 1964; p. 426. [Google Scholar]
- Tarabukina, E.B.; Simonova, M.A.; Bucatari, S.; Harabagiu, V.; Fundueanu, G.; Filippov, A.P. Behavior of thermo- and pH-responsive copolymer of N-isopropylacrylamide and maleic acid in aqueous solutions. Int. J. Polym. Anal. Charact. 2016, 21, 11–17. [Google Scholar] [CrossRef]
- Simonova, M.A.; Khayrullin, A.R.; Tyurina, V.O.; Kamorina, S.I.; Kamorin, D.M.; Sadikov, A.Y.; Filippov, A.P. Self-assembly processes in aqueous solutions of heatsensitive linear copolymers derived from n(dimethylamino)ethyl methacrylate. Fiber Chem. 2018, 50, 332–335. [Google Scholar] [CrossRef]
- Simonova, M.A.; Khayrullin, A.R.; Tyurina, V.O.; Kamorina, S.I.; Kamorin, D.M.; Sadikov, A.Y.; Filippov, A.P. Self-organization in aqueous solutions of thermosensitive statistical copolymers based on N-(dimethylamino)ethyl methacrylate. Int. J. Polym. Anal. Charact. 2019, 24, 630–638. [Google Scholar] [CrossRef]
- Zakharova, N.V.; Simonova, M.A.; Zelinskii, S.N.; Annenkov, V.V.; Filippov, A.P. Synthesis, molecular characteristics, and stimulus-sensitivity of graft copolymer of chitosan and poly (N,N-diethylacrylamide). J. Mol. Liquids 2019, 292, 111355. [Google Scholar] [CrossRef]







| Polymers | Ð | Mw∙10−3, g⋅mol−1 | Rh-D, nm | A2∙10−4, cm3∙mol∙g−2 | [η], cm3 g−1 | Rh-η, nm | dn/dc cm3∙g−1 |
|---|---|---|---|---|---|---|---|
| Chloroform | |||||||
| PDEAPMA-C | 1.8 * | 31/28 * | 3.9 | 1.9 | 0.07 | ||
| PDEAPMA-R | 1.3 | 36 | 4.4 | 4.6 | 0.08 | ||
| Water | |||||||
| PDEAPMA-C | 37 | 3.7 | 1.1 | 0.20 | |||
| PDEAPMA-R | 31 | 4.4 | 2.5 | 0.18 | |||
| Buffer (pH = 7) | |||||||
| PDEAPMA-C | 38 | 3.8 | 7.2 | 14.6 | 4.4 | 0.19 | |
| PDEAPMA-R | 31 | 4.0 | 6.5 | 14.2 | 4.1 | 0.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonova, M.; Kamorin, D.; Sadikov, A.; Filippov, A.; Kazantsev, O. The Influence of Synthesis Method on Characteristics of Buffer and Organic Solutions of Thermo- and pH-Responsive Poly(N-[3-(diethylamino)propyl]methacrylamide)s. Polymers 2022, 14, 282. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020282
Simonova M, Kamorin D, Sadikov A, Filippov A, Kazantsev O. The Influence of Synthesis Method on Characteristics of Buffer and Organic Solutions of Thermo- and pH-Responsive Poly(N-[3-(diethylamino)propyl]methacrylamide)s. Polymers. 2022; 14(2):282. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020282
Chicago/Turabian StyleSimonova, Maria, Denis Kamorin, Anton Sadikov, Alexander Filippov, and Oleg Kazantsev. 2022. "The Influence of Synthesis Method on Characteristics of Buffer and Organic Solutions of Thermo- and pH-Responsive Poly(N-[3-(diethylamino)propyl]methacrylamide)s" Polymers 14, no. 2: 282. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14020282