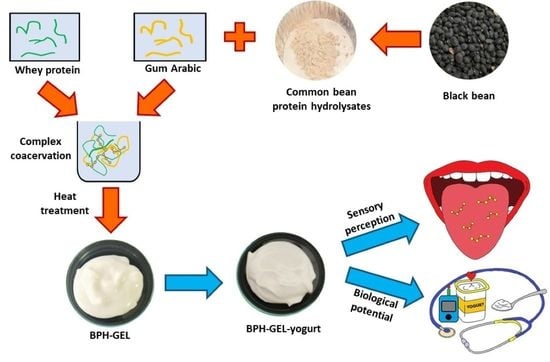
Sensory and Biological Potential of Encapsulated Common Bean Protein Hydrolysates Incorporated in a Greek-Style Yogurt Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel (W-GEL) Preparation
2.3. W-GEL Physicochemical Characterization
2.3.1. Yield (%)
2.3.2. Gel Strength (Firmness)
2.3.3. Syneresis
2.3.4. Rheological Measurement
2.4. Application of the W-GEL with BPH in a Greek-Style Yogurt
2.4.1. Common Bean Protein Hydrolysates (BPH)
2.4.2. Preparation of W-GEL with BPH (BPH-GEL)
2.4.3. Greek-Style Yogurt Preparation
2.5. Characterization of BPH-GEL-Yogurt and Control Yogurt
2.5.1. Syneresis
2.5.2. Titratable Acidity
2.5.3. Rheological Measurement
2.5.4. Confocal Microscopy of BPH-GEL and BPH-GEL-Yogurt
2.6. Sensory Evaluation of BPH-GEL-Yogurt and BPH-Ue-Yogurt
2.7. Biological Potential of BPH-GEL-Yogurt and Control Yogurt
2.7.1. Gastrointestinal Digestion In Vitro
2.7.2. α-Amylase Inhibition Assay
2.7.3. DPP-IV Inhibition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of W-GEL
3.2. Application of the W-GEL with BPH in a Greek-Style Yogurt
3.2.1. Characterization of BPH-GEL-Yogurt and Control Yogurt
3.2.2. Confocal Microscopy of BPH-GEL and BPH-GEL-Yogurt
3.3. Sensory Evaluation of BPH-GEL-Yogurt and BPH-Ue-Yogurt
3.4. Biological Potential
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| GA | Gum arabic |
| WPC | Whey protein concentrate |
| BPH | Common bean protein hydrolysates |
| W-GEL | Whey protein and gum arabic gel without common bean protein hydrolysates |
| BPH-GEL | Whey protein and gum arabic gel with encapsulated common bean protein hydrolysates |
| BPH-GEL-yogurt | Greek-style yogurt with encapsulated common bean protein hydrolysates in a whey protein and gum arabic gel. |
| Control yogurt | Greek-style yogurt without whey protein and gum arabic gel, and common bean protein hydrolysates |
| BPH-Ue-yogurt | Greek-style yogurt with unencapsulated common bean protein hydrolysates |
| DPP-IV | Dipeptidyl peptidase-4 |
| FITC | Fluorescein isothiocyanate |
| RBITC | Rhodamine isothiocyanate |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
References
- Birch, C.S.; Bonwick, G.A. Ensuring the future of functional foods. Int. J. Food Sci. Technol. 2019, 54, 1467–1485. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, L.; Wang, J.; Ma, A.; Fang, D.; Xiong, L.; Sun, Q. Construction of food-grade pH-sensitive nanoparticles for delivering functional food ingredients. Trends Food Sci. Technol. 2020, 96, 102–113. [Google Scholar] [CrossRef]
- Ramos-Lopez, A.; Mojica, L.; Gomez-Ojeda, A.; Macias-Cervantes, M.; Luevano-Contreras, C. Acute Effect of Black Bean (Phaseolus vulgaris L.) Hydrolyzed Protein on Glucose Levels in Adults with Prediabetes and Normal Glucose Tolerance. Curr. Dev. Nutr. 2020, 4, 458. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; Mojica, L.; de Mejía, E.G.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Characterization and comparison of protein and peptide profiles and their biological activities of improved common bean cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; de Mejia, E.G. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Mojica, L.; Chen, K.; de Mejía, E.G. Impact of commercial precooking of common bean (Phaseolus vulgaris) on the generation of peptides, after pepsin–pancreatin hydrolysis, capable to inhibit dipeptidyl peptidase-IV. J. Food Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; de Mejia, E.G. Black bean peptides inhibit glucose uptake in Caco-2 adenocarcinoma cells by blocking the expression and translocation pathway of glucose transporters. Toxicol. Rep. 2018, 5, 552–560. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, Q. Nanoencapsulation of functional food ingredients. Adv. Food Nutr. Res. 2019, 88, 129–165. [Google Scholar] [CrossRef]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- McClements, D.J. Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Nazir, A.; Asghar, A.; Maan, A.A. Food gels: Gelling process and new applications. In Advances in Food Rheology and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 335–353. [Google Scholar]
- Azarikia, F.; Wu, B.-C.; Abbasi, S.; McClements, D.J. Stabilization of biopolymer microgels formed by electrostatic complexation: Influence of enzyme (laccase) cross-linking on pH, thermal, and mechanical stability. Food Res. Int. 2015, 78, 18–26. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; Fernandes, R.V.D.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Kuhn, K.R.; e Silva, F.G.D.; Netto, F.M.; da Cunha, R.L. Production of whey protein isolate–gellan microbeads for encapsulation and release of flaxseed bioactive compounds. J. Food Eng. 2019, 247, 104–114. [Google Scholar] [CrossRef]
- Newman, J.; O’Riordan, D.; Jacquier, J.; O’Sullivan, M. Masking of bitterness in dairy protein hydrolysates: Comparison of an electronic tongue and a trained sensory panel as means of directing the masking strategy. LWT-Food Sci. Technol. 2015, 63, 751–757. [Google Scholar] [CrossRef]
- Silva, D.; Favaro-Trindade, C.; Rocha, G.; Thomazini, M. Microencapsulation of lycopene by gelatin–pectin complex coacervation. J. Food Process. Preserv. 2012, 36, 185–190. [Google Scholar] [CrossRef]
- Breternitz, N.R.; Bolini, H.M.A.; Hubinger, M.D. Sensory acceptance evaluation of a new food flavoring produced by microencapsulation of a mussel (Perna perna) protein hydrolysate. LWT-Food Sci. Technol. 2017, 83, 141–149. [Google Scholar] [CrossRef]
- Mendanha, D.V.; Ortiz, S.E.M.; Favaro-Trindade, C.S.; Mauri, A.; Monterrey-Quintero, E.S.; Thomazini, M. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 2009, 42, 1099–1104. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Available online: www.diabetes.org/ (accessed on 9 August 2021).
- Gyawali, R.; Ibrahim, S.A. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.-O.; Hoffmann, T.K.; Johansen, A.-G.; Skeie, S.B. Processing of high-protein yoghurt–A review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Godoy-García, L.; Abadía-García, L.; Cruz-Aldaco, K.; Castaño-Tostado, E.; Murúa-Pagola, B.; Amaya-Llano, S.L. Addition of glycomacropeptide as fat replacer in sugar-reduced Greek-style yoghurt. Int. J. Dairy Technol. 2020, 73, 718–725. [Google Scholar] [CrossRef]
- Eleya, M.O.; Turgeon, S. The effects of pH on the rheology of β-lactoglobulin/κ-carrageenan mixed gels. Food Hydrocoll. 2000, 14, 245–251. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Morales-Hernández, N.; Lobato-Calleros, C.; Vernon-Carter, E.J. Mesquite gum/chitosan insoluble complexes: Relationship between the water state and viscoelastic properties. J. Dispers. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Qin, X.S.; Luo, S.Z.; Cai, J.; Zhong, X.Y.; Jiang, S.T.; Zhao, Y.Y.; Zheng, Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016, 31, 590–597. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Compressive textural attributes, opacity and syneresis of gels prepared from gellan, agar and their mixtures. J. Food Eng. 2011, 102, 287–292. [Google Scholar] [CrossRef]
- Li, F.; Kong, X.; Zhang, C.; Hua, Y. Gelation behaviour and rheological properties of acid-induced soy protein-stabilized emulsion gels. Food Hydrocoll. 2012, 29, 347–355. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT-Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- NOM-243-SSA1. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos—Disposiciones y Especificaciones Sanitarias—Métodos de Prueba. 2010. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5160755&fecha=27/09/2010#:~:text=1.1%20Esta%20Norma%20Oficial%20Mexicana,combinado%20y%20los%20derivados%20l%C3%A1cteos (accessed on 18 October 2021).
- Schmitt, C.; Sanchez, C.; Lamprecht, A.; Renard, D.; Lehr, C.-M.; de Kruif, C.G.; Hardy, J. Study of β-lactoglobulin/acacia gum complex coacervation by diffusing-wave spectroscopy and confocal scanning laser microscopy. Colloids Surf. B Biointerfaces 2001, 20, 267–280. [Google Scholar] [CrossRef]
- ISO (International Organization for Standarization). Sensory analysis—General guidance for the selection, training and monitoring of assessors. In Part 2: Expert Sensory Assessors; ISO 8586-2: Geneva, Switzerland, 2008. [Google Scholar]
- Sanguansri, L.; Day, L.; Shen, Z.; Fagan, P.; Weerakkody, R.; Cheng, L.J.; Rusli, J.; Augustin, M.A. Encapsulation of mixtures of tuna oil, tributyrin and resveratrol in a spray dried powder formulation. Food Funct. 2013, 4, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Kusano, R.; Ogawa, S.; Matsuo, Y.; Tanaka, T.; Yazaki, Y.; Kouno, I. α-Amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J. Nat. Prod. 2011, 74, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo-Herrera, G.; Mojica, L. Black bean anthocyanin-rich extract from supercritical and pressurized extraction increased in vitro antidiabetic potential, while having similar storage stability. Foods 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; McClements, D.J. Structure–function relationships in food emulsions: Improving food quality and sensory perception. Food Struct. 2014, 1, 106–126. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Emulsifying properties of soy protein nanoparticles: Influence of the protein concentration and/or emulsification process. J. Agric. Food Chem. 2014, 62, 2644–2654. [Google Scholar] [CrossRef]
- Mao, R.; Tang, J.; Swanson, B. Water holding capacity and microstructure of gellan gels. Carbohydr. Polym. 2001, 46, 365–371. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Sandoval-Castilla, O.; Vázquez-Torres, H.; Vernon-Carter, E.J.; Lobato-Calleros, C. Determination of the gum Arabic–chitosan interactions by Fourier Transform Infrared Spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohydr. Polym. 2010, 79, 541–546. [Google Scholar] [CrossRef]
- Weinbreck, F.; Minor, M.; De Kruif, C. Microencapsulation of oils using whey protein/gum arabic coacervates. J. Microencapsul. 2004, 21, 667–679. [Google Scholar] [CrossRef]
- Rojas-Moreno, S.; Cardenas-Bailon, F.; Osorio-Revilla, G.; Gallardo-Velazquez, T.; Proal-Najera, J. Effects of complex coacervation-spray drying and conventional spray drying on the quality of microencapsulated orange essential oil. J. Food Meas. Charact. 2018, 12, 650–660. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Vicente, J.; dos Santos, C.H.C.; de Garvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P. Microencapsulation of palm oil by complex coacervation for application in food systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Pimentel, T.C.; Guimaraes, J.T.; Balthazar, C.F.; Rocha, R.S.; Cavalcanti, R.N.; Esmerino, E.A.; Freitas, M.Q.; Raices, R.S.; Silva, M.C. Impact of prebiotics on the rheological characteristics and volatile compounds of Greek yogurt. LWT 2019, 105, 371–376. [Google Scholar] [CrossRef]
- Pinto, S.S.; Cavalcante, B.D.; Verruck, S.; Alves, L.F.; Prudêncio, E.S.; Amboni, R.D. Effect of the incorporation of Bifidobacterium BB-12 microencapsulated with sweet whey and inulin on the properties of Greek-style yogurt. J. Food Sci. Technol. 2017, 54, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Shepard, L.; Drake, M. Sensory properties and drivers of liking for Greek yogurts. J. Dairy Sci. 2013, 96, 7454–7466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uduwerella, G.; Chandrapala, J.; Vasiljevic, T. Preconcentration of yoghurt base by ultrafiltration for reduction in acid whey generation during Greek yoghurt manufacturing. Int. J. Dairy Technol. 2018, 71, 71–80. [Google Scholar] [CrossRef]
- Uduwerella, G.; Chandrapala, J.; Vasiljevic, T. Minimising generation of acid whey during Greek yoghurt manufacturing. J. Dairy Res. 2017, 84, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Villeda, C.M. Elaboración de Yogur Estilo Griego con Diferentes Porcentajes de ATECAL, Leche en Polvo y Horas de Desuerado. Bachelor’s Thesis, Escuela Agricola Panamericana, Zamorano, Tegucigalpa, Honduras, 2015. [Google Scholar]
- NOM-181-SCFI/SAGARPA. Yogurt—Denominación, Especificaciones Fisicoquímicas y Microbiológicas, Información Comercial y Métodos de Prueba. 2018. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5549317&fecha=31/01/2019 (accessed on 18 October 2021).
- Fernandez, M.A.; Picard-Deland, É.; Barz, M.L.; Daniel, N.; Marette, A. Yogurt and Health. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–338. [Google Scholar]
- Rinaldoni, A.N.; Campderrós, M.E.; Padilla, A.P. Physico-chemical and sensory properties of yogurt from ultrafiltreted soy milk concentrate added with inulin. LWT-Food Sci. Technol. 2012, 45, 142–147. [Google Scholar] [CrossRef]
- Gilbert, A.; Turgeon, S.L. Studying stirred yogurt microstructure and its correlation to physical properties: A review. Food Hydrocoll. 2021, 106970. [Google Scholar] [CrossRef]
- Oliveira, M.; Sodini, I.; Remeuf, F.; Corrieu, G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int. Dairy J. 2001, 11, 935–942. [Google Scholar] [CrossRef]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-based coacervates: Structures, functionality and applications in food products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.; Moraes, I.C.; Pinho, S.C. Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. LWT 2017, 86, 166–173. [Google Scholar] [CrossRef]
- Estrada-Fernández, A.; Román-Guerrero, A.; Jiménez-Alvarado, R.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E. Stabilization of oil-in-water-in-oil (O1/W/O2) Pickering double emulsions by soluble and insoluble whey protein concentrate-gum Arabic complexes used as inner and outer interfaces. J. Food Eng. 2018, 221, 35–44. [Google Scholar] [CrossRef]
- Mishra, P.K.; Tripathi, J.; Gupta, S.; Variyar, P.S. Effect of cooking on aroma profile of red kidney beans (Phaseolus vulgaris) and correlation with sensory quality. Food Chem. 2017, 215, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Erami, S.R.; Amiri, Z.R.; Jafari, S.M. Nanoliposomal encapsulation of Bitter Gourd (Momordica charantia) fruit extract as a rich source of health-promoting bioactive compounds. LWT 2019, 116, 108581. [Google Scholar] [CrossRef]
- Mojica, L.; De Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yu, T.; He, J.; Cui, J.; Wang, J.; Cheng, X.; Fan, J. Oat globulin peptides regulate antidiabetic drug targets and glucose transporters in Caco-2 cells. J. Funct. Foods 2018, 42, 12–20. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Mocan, A.; Macedonio, G.; Carradori, S.; Onaolapo, O.; Onaolapo, A.; Adegoke, J. Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: In vivo and in vitro evaluation of its nutraceutical potential. J. Funct. Foods 2017, 35, 32–42. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef]
- Shori, A.B. Proteolytic activity, antioxidant, and α-Amylase inhibitory activity of yogurt enriched with coriander and cumin seeds. LWT 2020, 133, 109912. [Google Scholar] [CrossRef]





| Tx. | Concentration | Ration. | Yield | Gel Strength | Syneresis | Rheology G′(Pa) | |
|---|---|---|---|---|---|---|---|
| (%) | (WPC-GA) | (%) | (gf) | (%) | Ɯ (0.1) | Ɯ (100) | |
| W-GEL 1 | 7.5 | 5:1 | 36.56 ± 1.17 a | 55 ± 2 a | 4.20 ± 0.05 a | 5722 a | 13,490 a |
| W-GEL 2 | 7.5 | 3:1 | 35.78 ± 1.35 a | 96 ± 5 b | 2.71 ± 0.25 b | 3753 b | 9044 b |
| W-GEL3 | 5.0 | 5:1 | 35.33 ± 1.89 a | 159 ± 8 c | 3.89 ± 0.62 a | 5473 a | 13,680 a |
| W-GEL 4 | 5.0 | 3:1 | 39.33 ± 1.76 a | 100 ± 2 b | 0.37 ± 0.25 c | 5508 a | 13,730 a |
| Treatment | Sample | α-Amylase Inhibition | DPP-IV Inhibition |
|---|---|---|---|
| (%) | (%) | ||
| BPH-GEL-yogurt | Initial | 1.55 ± 1.61 aA | ND |
| Stomach | 2.90 ± 0.18 aA | ND | |
| Intestine | 92.47 ± 0.58 bA | 75.24 ± 1.43 A | |
| Control yogurt | Initial | 0.39 ± 4.37 aA | ND |
| Stomach | 0.063 ± 3.5 aA | ND | |
| Intestine | 89.03 ± 0.97 bB | 69.13 ± 0.74 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Free-Manjarrez, S.; Mojica, L.; Espinosa-Andrews, H.; Morales-Hernández, N. Sensory and Biological Potential of Encapsulated Common Bean Protein Hydrolysates Incorporated in a Greek-Style Yogurt Matrix. Polymers 2022, 14, 854. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050854
Free-Manjarrez S, Mojica L, Espinosa-Andrews H, Morales-Hernández N. Sensory and Biological Potential of Encapsulated Common Bean Protein Hydrolysates Incorporated in a Greek-Style Yogurt Matrix. Polymers. 2022; 14(5):854. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050854
Chicago/Turabian StyleFree-Manjarrez, Samantha, Luis Mojica, Hugo Espinosa-Andrews, and Norma Morales-Hernández. 2022. "Sensory and Biological Potential of Encapsulated Common Bean Protein Hydrolysates Incorporated in a Greek-Style Yogurt Matrix" Polymers 14, no. 5: 854. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14050854