Extraction Optimization of Mucilage from Seeds of Mimosa pudica by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Mucilage
2.2.2. Calculation of Yield
2.2.3. Experimental Design and Statistical Analysis
2.2.4. Evaluation of MPM as a Sustained Release Material
Preparation of MPM-Based Tablets
pH-Responsive On–Off Switching Studies
In Vitro Release Study of Itopride
Drug Release Kinetics and Mechanism
3. Results and Discussion
3.1. Extraction of Mucilage
3.2. Preliminary Studies for the Extraction Optimization of MPM Yield
3.2.1. Effect of pH
3.2.2. Effect of Seed/Water Contact Time
3.2.3. Effect of Temperature
3.2.4. Effect of Seed/Water Ratio
3.3. Fitting of Model
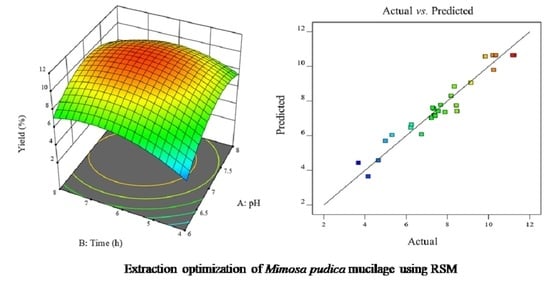
3.4. Interpretation of Response Surface Plots
3.5. Model Adequacy
3.6. Optimization of Extraction Yield and Checking of Model Desirability
3.7. Comparison of Extraction Yield of MPM with Already Reported Mucilages
3.8. pH-Responsive On–Off Switching Studies
3.9. In Vitro Release Study of Itopride
3.10. Applications and Future Research Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junior, F.A.L.; Conceição, M.C.; de Resende, J.V.; Junqueira, L.A.; Pereira, C.G.; Prado, M.E.T. Response surface methodology for optimization of the mucilage extraction process from Pereskia aculeata Miller. Food Hydrocoll. 2013, 33, 38–47. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.E.; Dias Ruivo, T.; da Silva Scapim, M.R.; Madrona, G.S.; de Bergamasco, R.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Keshani-Dokht, S.; Emam-Djomeh, Z.; Yarmand, M.S.; Fathi, M. Extraction, chemical composition, rheological behavior, antioxidant activity and functional properties of Cordia myxa mucilage. Int. J. Biol. Macromol. 2018, 118, 485–493. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, C.; Feng, X.; Lu, B.; Xia, L.; Zhuang, X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption-photocatalytic removal of Congo red. Carbohydr. Polym. 2022, 288, 119332. [Google Scholar] [CrossRef]
- Yu, Z.F.; Song, S.; Xu, X.L.; Ma, Q.; Lu, Y. Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Irfan, J.; Hussain, M.A.; Haseeb, M.T.; Ali, A.; Farid-ul-Haq, M.; Tabassum, T.; Hussain, S.Z.; Hussain, I.; Naeem-ul-Hassan, M. A pH-sensitive, stimuli-responsive, superabsorbent, smart hydrogel from psyllium (Plantago ovata) for intelligent drug delivery. RSC Adv. 2021, 11, 19755–19767. [Google Scholar] [CrossRef]
- Soltani, M.D.; Meftahizadeh, H.; Barani, M.; Rahdar, A.; Hosseinikhah, S.M.; Hatami, M.; Ghorbanpour, M. Guar (Cyamopsis tetragonoloba L.) plant gum: From biological applications to advanced nanomedicine. Int. J. Biol. Macromol. 2021, 193, 1972–1985. [Google Scholar] [CrossRef]
- Qing, W.; Xinmin, W.; Shuo, P. The three-dimensional molecular structure model of Fushun oil shale kerogen, China. J. Mol. Struct. 2022, 1255, 132380. [Google Scholar] [CrossRef]
- Muhammad, G.; Hussain, M.A.; Jantan, I.; Bukhari, S.N.A. Mimosa pudica L., a high-value medicinal plant as a source of bioactives for pharmaceuticals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, R.; Pokharkar, R. GCMS studies of Mimosa pudica. Int. J. PharmTech Res. 2012, 4, 93–98. [Google Scholar]
- Rezagholi, F.; Hashemi, S.M.B.; Gholamhosseinpour, A.; Sherahi, M.H.; Hesarinejad, M.A.; Ale, M.T. Characterizations and rheological study of the purified polysaccharide extracted from quince seeds. J. Sci. Food Agric. 2019, 99, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, G.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Hussain, S.Z.; Hussain, I. Polysaccharide based superabsorbent hydrogel from Mimosa pudica: Swelling-deswelling and drug release. RSC Adv. 2016, 6, 23310–23317. [Google Scholar] [CrossRef]
- Muhammad, G.; Ashraf, M.U.; Naeem-ul-Hassan, M.; Bukhari, S.N.A. Appraisal of acute oral toxicity of glucuronoxylan hydrogel from Mimosa pudica seeds. Braz. J. Pharm. Sci. 2018, 54, e17579. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Mazza, G.; Oomah, B.D.; Biliaderis, C.G. Optimization of an aqueous extraction process for flaxseed gum by response surface methodology. LWT Food Sci. Technol. 1994, 27, 363–369. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Cheng, S.; He, F.; Fu, L.; Zhang, Y. Polysaccharide from rubescens: Extraction, optimization, characterization and antioxidant activities. RSC Adv. 2021, 11, 18974–18983. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Chen, L.; Zhao, G.; Mi, Z.; Lv, D.; Niu, J. Optimizing the extraction of polysaccharides from Bletilla ochracea Schltr. using response surface methodology (RSM) and evaluating their antioxidant activity. Processes 2020, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Tahmouzi, S. Optimization of polysaccharides from Zagros oak leaf using RSM: Antioxidant and antimicrobial activities. Carobohydr. Polym. 2014, 106, 238–246. [Google Scholar] [CrossRef]
- Sui, Z.; Li, L.; Liu, B.; Gu, T.; Zhao, Z.; Liu, C.; Shi, C.; Yang, R. Optimum conditions for Radix Rehmanniae polysaccharides by RSM and its antioxidant and immunity activity in UVB mice. Carbohydr. Polym. 2013, 92, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations- theoretical considerations and application to non-disintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropylmethylcellulose. Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Somboonpanyakul, P.; Wang, Q.; Cui, W.; Barbut, S.; Jantawat, P. Malva nut gum. (Part I): Extraction and physicochemical characterization. Carbohydr. Polym. 2006, 64, 247–253. [Google Scholar] [CrossRef]
- Balke, D.T.; Diosady, L.L. Rapid aqueous extraction of mucilage from whole white mustard seed. Food Res. Int. 2000, 33, 347–356. [Google Scholar] [CrossRef]
- Estévez, A.M.; Saenz, C.; Hurtado, M.L.; Escobar, B.; Espinoza, S.; Suárez, C. Extraction methods and some physical properties of mesquite (Prosopis chilensis (Mol) Stuntz) seed gum. J. Sci. Food Agric. 2004, 84, 1487–1492. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A.; Masoodi, F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction optimization, purification, antioxidant activity, and preliminary structural characterization of crude polysaccharide from an Arctic Chlorella sp. Polymers 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.L.; Jiang, C.J. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr. Polym. 2011, 84, 495–502. [Google Scholar] [CrossRef]
- Singh, B.; Oberoi, D.P.S.; Wani, I.A.; Sogi, D.S. Effect of temperature, salt concentration, pH and time on thermal degradation of pumpkin (Cucurbita pepo) puree. Adv. Food Sci. 2009, 31, 96–101. [Google Scholar]
- Koocheki, A.; Mortazavi, S.A.; Shahidi, F.; Razavi, S.M.A.; Taherian, A.R. Rheological properties of mucilage extracted from Alyssum homolocarpum seed as a new source of thickening agent. J. Food Eng. 2009, 91, 490–496. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Razavi, S.M.; Bostan, A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll. 2009, 23, 2369–2379. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int. J. Biol. Macromol. 2014, 66, 113–124. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A. Optimization of extraction process of carob bean gum purified from carob seeds by response surface methodology. Chem. Process Eng. Res. 2013, 12, 1–8. [Google Scholar]
- Bendahou, A.; Dufresne, A.; Kaddami, H.; Habibi, Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydr. Polym. 2007, 68, 601–608. [Google Scholar] [CrossRef]
- Joglekar, A.M.; May, A.T. Optimization of multigrain premix for high protein and dietary fibre biscuits using response surface methodology (RSM). Cereal Food World 1987, 32, 857–868. [Google Scholar]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, R.C. Response Surface Methodology, Process and Product Optimization Using Design Experiment; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Amin, M.A.; Ahmad, A.S.; Yinyin, Y.; Yahya, N.; Ibrahim, N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocoll. 2007, 21, 273–279. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Bostan, A. Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2012, 50, 446–456. [Google Scholar] [CrossRef]
- Ali, A.; Hussain, M.A.; Haseeb, M.T.; Bukhari, S.N.A.; Tabassum, T.; Farid-ul-Haq, M.; Sheikh, F.A. A pH-responsive, biocompatible, and non-toxic citric acid cross-linked polysaccharide-based hydrogel from Salvia spinosa L. offering zero-order drug release. J. Drug Deliv. Sci. Technol. 2022, 69, 103144. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Facile preparation of pH-sensitive chitosan microspheres for delivery of curcumin; Characterization, drug release kinetics and evaluation of anticancer activity. Int. J. Biol. Macromol. 2020, 162, 501–511. [Google Scholar] [CrossRef]
- Cok, M.; Viola, M.; Vecchies, F.; Sacco, P.; Furlani, F.; Marsich, E.; Donati, I. N-isopropyl chitosan. A pH- and thermo-responsive polysaccharide for gel formation. Carbohydr. Polym. 2020, 230, 115641. [Google Scholar] [CrossRef]
- Lodhi, B.A.; Hussain, M.A.; Sher, M.; Haseeb, M.T.; Ashraf, M.U.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A. Polysaccharide-based superporous, superabsorbent, and stimuli responsive hydrogel from sweet basil: A novel material for sustained drug release. Adv. Polym. Technol. 2019, 2019, 9583516. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, B.A.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Muhammad, G.; Farid-ul-Haq, M.; Naeem-ul-Hassan, M. Basil (Ocimum basilicum L.) seeds engender a smart material for intelligent drug delivery: On-off switching and real-time swelling, in vivo transit detection, and mechanistic studies. Ind. Crop. Prod. 2020, 155, 112780. [Google Scholar] [CrossRef]






| Constituents of the Tablets | F1 | F2 | F3 |
|---|---|---|---|
| MPM | 75 | 100 | 125 |
| Itopride | 100 | 100 | 100 |
| Microcrystalline cellulose | 120 | 95 | 70 |
| Magnesium stearate | 5 | 5 | 5 |
| Total weight | 300 | 300 | 300 |
| Run Nos. | Independent Variables | Yield (%) | ||||
|---|---|---|---|---|---|---|
| pH A | Seed/Water Contact Time (h) B | Extraction Temperature (°C) C | Seed/Water Ratio (w/v) D | Actual Y | Predicted Z | |
| 1 | 7 | 6 | 30 | 10 | 8.44 | 7.76 |
| 2 | 6 | 6 | 30 | 20 | 4.16 | 3.66 |
| 3 | 8 | 6 | 70 | 20 | 7.23 | 7.03 |
| 4 | 7 | 4 | 30 | 20 | 5.32 | 6.05 |
| 5 | 7 | 6 | 50 | 20 | 11.25 | 10.66 |
| 6 | 7 | 4 | 70 | 20 | 6.26 | 6.65 |
| 7 | 7 | 8 | 70 | 20 | 9.15 | 9.06 |
| 8 | 7 | 6 | 70 | 10 | 6.75 | 6.08 |
| 9 | 8 | 6 | 50 | 10 | 8.34 | 8.85 |
| 10 | 7 | 8 | 50 | 30 | 10.25 | 9.81 |
| 11 | 7 | 8 | 30 | 20 | 6.23 | 6.47 |
| 12 | 8 | 6 | 50 | 30 | 7.55 | 7.44 |
| 13 | 8 | 6 | 30 | 20 | 7.89 | 7.37 |
| 14 | 7 | 8 | 50 | 10 | 7.28 | 7.62 |
| 15 | 8 | 8 | 50 | 20 | 7.68 | 7.78 |
| 16 | 7 | 4 | 50 | 10 | 7.41 | 7.16 |
| 17 | 6 | 6 | 50 | 30 | 8.19 | 8.31 |
| 18 | 7 | 4 | 50 | 30 | 8.46 | 7.43 |
| 19 | 6 | 6 | 70 | 20 | 7.38 | 7.20 |
| 20 | 7 | 6 | 70 | 30 | 9.85 | 10.59 |
| 21 | 8 | 4 | 50 | 20 | 7.34 | 7.55 |
| 22 | 7 | 6 | 30 | 30 | 4.99 | 5.72 |
| 23 | 7 | 6 | 50 | 20 | 10.36 | 10.66 |
| 24 | 7 | 6 | 50 | 20 | 11.17 | 10.66 |
| 25 | 6 | 4 | 50 | 20 | 4.64 | 4.59 |
| 26 | 7 | 6 | 50 | 20 | 10.31 | 10.66 |
| 27 | 6 | 8 | 50 | 20 | 7.35 | 7.20 |
| 28 | 6 | 6 | 50 | 10 | 3.68 | 4.43 |
| 29 | 7 | 6 | 50 | 20 | 10.21 | 10.66 |
| Source | Sum of Squares | DF a | Mean | F-Value | p-Value b,c |
|---|---|---|---|---|---|
| Model | 110.10 | 14 | 7.86 | 16.39 | <0.0001 ** |
| Linear | 9.42 | 1 | 9.42 | 19.62 | 0.0006 ** |
| A-pH | 6.04 | 1 | 6.04 | 12.57 | 0.0032 ** |
| B—Contact time (h) | 7.66 | 1 | 7.66 | 15.97 | 0.0013 ** |
| C—Temperature (°C) | 4.55 | 1 | 4.55 | 9.48 | 0.0082 ** |
| D—Seed/water ratio | 110.10 | 14 | 7.86 | 16.39 | <0.0001 ** |
| Quadratic | |||||
| A2 | 34.63 | 1 | 34.63 | 72.15 | <0.0001 ** |
| B2 | 15.95 | 1 | 15.95 | 33.23 | <0.0001 ** |
| C2 | 26.81 | 1 | 26.81 | 55.86 | <0.0001 ** |
| D2 | 7.71 | 1 | 7.71 | 16.07 | 0.0013 ** |
| Interaction | |||||
| AB | 1.40 | 1 | 1.40 | 2.93 | ns |
| AC | 3.76 | 1 | 3.76 | 7.84 | 0.0142 * |
| AD | 7.02 | 1 | 7.02 | 14.63 | 0.0019 ** |
| BC | 0.9801 | 1 | 0.9801 | 2.04 | ns |
| BD | 0.9216 | 1 | 0.9216 | 1.92 | ns |
| CD | 10.73 | 1 | 10.73 | 22.35 | 0.0003 ** |
| Residual | 6.72 | 14 | 0.4799 | ||
| Lack of Fit | 5.70 | 10 | 0.5696 | 2.23 | ns |
| Pure Error | 1.02 | 4 | 0.2558 | ||
| Cor Total | 116.82 | 28 | |||
| R2 = 0.94255; Adjusted-R2 = 0.8850; Predicted-R2 = 0.7055; CV = 8.92%; Mean = 7.76; ADP = 14.0462 | |||||
| Formulation Code | Zero-Order Kinetic Model | Korsmeyer-Peppas Model | |||
|---|---|---|---|---|---|
| R2 | K0 | R2 | KKP | n | |
| F3 | 0.9907 | 6.758 | 0.9933 | 8.178 | 0.915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhari, S.N.A.; Ali, A.; Hussain, M.A.; Tayyab, M.; Alotaibi, N.F.; Elsherif, M.A.; Junaid, K.; Ejaz, H. Extraction Optimization of Mucilage from Seeds of Mimosa pudica by Response Surface Methodology. Polymers 2022, 14, 1904. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091904
Bukhari SNA, Ali A, Hussain MA, Tayyab M, Alotaibi NF, Elsherif MA, Junaid K, Ejaz H. Extraction Optimization of Mucilage from Seeds of Mimosa pudica by Response Surface Methodology. Polymers. 2022; 14(9):1904. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091904
Chicago/Turabian StyleBukhari, Syed Nasir Abbas, Arshad Ali, Muhammad Ajaz Hussain, Muhammad Tayyab, Nasser F. Alotaibi, Mervat A. Elsherif, Kashaf Junaid, and Hasan Ejaz. 2022. "Extraction Optimization of Mucilage from Seeds of Mimosa pudica by Response Surface Methodology" Polymers 14, no. 9: 1904. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14091904