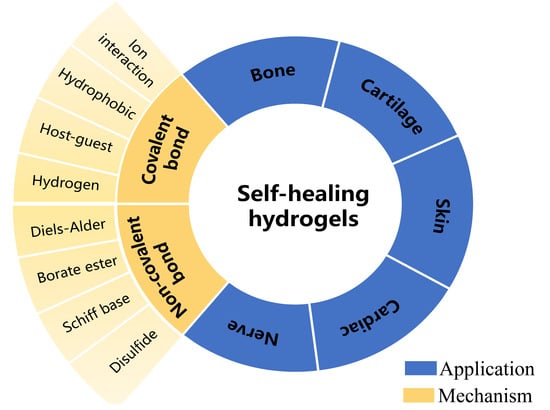
1. Introduction
Hydrogels with adjustable physical and chemical properties are composed of 3D polymeric networks [
1]. Hydrogels are similar to the structure and function of the extracellular matrix, which can absorb and retain a large amount of water without dissolving in a swelling state [
2]. Therefore, hydrogels have long been widely used in tissue repair, drug delivery, and other fields [
3,
4,
5]. Due to the complex dynamic physiological and mechanical environment inside the human body, the hydrogel will appear with minor defects after implantation. Due to the irreversibility of the conventional hydrogel after destruction, these minor defects will gradually increase and merge into large cracks, resulting in material failure [
6,
7,
8]. Consequently, to improve the practical life of implanted hydrogel, it is necessary to design a self-healing hydrogel.
Self-healing material is defined as the material that can heals and restores damage automatically. Self-healing hydrogels can achieve self-healing through external stimuli (light, heat, pH) or the interaction of mutual covalent and noncovalent bonds of functional groups in hydrogels after damage [
9,
10,
11]. The self-healing properties of self-healing hydrogels improve the fatal shortcomings of hydrogels that cannot recover spontaneously after damage, greatly promote the development of hydrogels to multifunctional composite hydrogels, and further broaden the application of hydrogels in the field of biomedicine.
The related research on self-healing hydrogels is increasing. The self-healing mechanisms of self-healing hydrogels of different researchers and the application directions of hydrogels are also different. In this review, we review the research results of self-healing hydrogels in recent years according to the self-healing mechanism of hydrogels. Then the different applications of self-healing hydrogels in tissue engineering were summarized, and the functional factors of self-healing hydrogels in various applications were generalized. We hope that the summary of this paper can provide a theoretical reference for researchers to develop more novelty-specialized intelligent hydrogels.
2. Mechanism of Self-Healing Hydrogels
The self-healing mechanisms of hydrogels are mainly based on the reversibility of their cross-linking structures, which often involve dynamic covalent bonds [
1,
2] (such as Schiff base bond, borate ester bond [
3], Diels–Alder reaction [
4], and disulfide bond [
5] and dynamic noncovalent bond interactions (such as hydrogen bond interaction [
6], ion interaction (metal coordination) [
7,
8], host–guest interaction [
9,
10], hydrophobic interaction [
11]). Hydrogels’ stability, self-healing ability, and mechanical properties are directly related to the number and strength (or type) of chemical bonds used in the synthesis of hydrogels. Therefore, researchers need to understand the self-healing mechanism of different hydrogels to design hydrogels with varying self-healing effects.
The covalently cross-linked hydrogel polymer network synthesized by the traditional chemical method is irreversible and easy to be fatigued or damaged in use. Because the dynamic covalent bond is reversible as a process of ‘fracture-formation’ in a mild environment, it is often used to construct self-healing hydrogels. Dynamic noncovalent bond interaction is a kind of physical interaction with natural reversibility and stability of dynamic fracture recombination. It is a good choice for designing self-healing hydrogel.
2.1. Schiff Base
German chemist Hugo Schiff discovered the imine bond in 1864, so amine-based compounds are usually called “Schiff bases” [
12]. Schiff base is considered a strong covalent bond (150 kcal mol
−1) [
13], including imine (
Figure 1a), oximes (
Figure 1b), hydrazones (
Figure 1c) and hydrazine (
Figure 1d). In Schiff base reaction, the amine nitrogen (a nucleophile) attacks the electrophile carbonyl atom in the aldehyde to yield a double nitrogen–carbon bond (Schiff base). In addition, the Schiff base can still go back to amines and the functional carbonyl groups through hydrolysis. Consequently, the Schiff base reaction may form a dynamic equilibrium under physiological conditions. In recent years, Schiff bases, as a class of chemical bonds capable of dynamic reversible fracture recombination, have been widely used in hydrogel networks to construct self-healing hydrogels [
14]. The acylhydrazone bond is usually synthesized by the condensation reaction of hydrazine and aldehyde [
15,
16]. The acylhydrazone bond is exceptionally similar to the imide bond, but the acylhydrazone bond can spontaneously form under physiological conditions (the rate is much lower than that in an acidic environment) [
16,
17]. Polymers possess amino-rich such as chitosan and polyethyleneimine, combine with polymers containing aldehyde and ketone groups, and obtain unique self-healing properties by the dynamic reversibility of imide and acylhydrazone bonds.
ε-poly(L-lysine) carbon dot (PL-CD) with a large number of amino groups on the surface and oxidized dextran (ODA) were cross-linked by Schiff base to form hydrogels [
18]. In addition to excellent self-healing properties, PL-CD also had excellent antibacterial properties. As the contacting and releasing antibacterial action of the PL-CD@ODA hydrogel, 10% CFU/mL of 10
7 S. aureus was killed after 10 min of contact. In addition, PL-CD@ODA hydrogels showed flexible injection and strong self-healing properties after severe damage. Complete healing can be achieved in a few seconds when 1000% shear stress is applied to the hydrogel. Baolin Guo et al. [
19] successfully synthesized injectable self-healing conductive hydrogel composed of dextran grafted aniline tetramer-grafted-4-formylbenzoic acid (Dex-AT-FA) and
N-carboxyethyl chitosan (CECS). Grafted aniline oligomers provide sufficient electrical activity and conductivity for hydrogels (10
−2 ms/cm); dynamic cross-linked network formed by Schiff base bond provides excellent self-healing ability for hydrogels. The hydrogel with enough in vivo injection and degradation can encapsulate different types of cells, and the released cells still show continuous proliferation ability.
2.2. Borate Ester Bond
Boronic acid can accept electron pairs; it can form complexes with hydroxide or electron-donating groups containing oxygen or nitrogen atoms. In an aqueous solution, boronic acid co-exists in its neutral state or is bound to a hydroxide ion, and its sp
2 and sp
3 hybrid orbitals adopt trigonal planar and tetragonal geometries. Therefore, Boronic acid forms a borate ester bond with 1,2-diols or 1,3-diols in an aqueous solution or in its bulk form or an organic solvent [
20]. The dynamic equilibrium of borate ester bond reversibly formed or broken is affected by pH, heat, aqueous media and some biomolecules. For this reason, boronic acid can be applied to sensors that can selectively detect the biomolecules including saccharides, such as glucose [
21,
22], or can be used as a component in the drug delivery systems [
23,
24], and self-healing materials.
Phenylboronic acid and cis-diol-modified polyethylene glycol form boric acid ester bonds to cross-link into pH-responsive hydrogels [
25]. These gels also exhibit size-dependent controlled release of proteins encapsulated within the network and the glucose-responsive release of larger proteins.
Dynamic borate hydrogel can also improve the bioavailability of poorly water-soluble drugs (such as curcumin) [
26]. Ruihao Pan et al. developed a multi-responsive self-healing hydrogel for the controlled release of curcumin by using a dynamic borate ester bond. Hydrogels were synthesized by coupling curcumin with polyvinyl alcohol using a polymer containing phenylboronic acid. The synthesized curcumin-loaded hydrogels were self-healing due to the dynamic nature of borate bonds; the variety of stimuli can change the equilibrium transfer between borate and boric acid, thereby affecting the release rate of curcumin to achieve drug-controlled release.
2.3. Disulfide Bond
The disulfide bonds (R–S–S–R) plays a main role in the folding of cysteine residues to protein [
27,
28]. Therefore, disulfide bonds are widely distributed in nature. Thiol-disulfide exchange reactions can occur between thiolates and disulfides. In addition, thiols can be oxidized to disulfides, and disulfides can be reduced to thiols. Thus, the reaction that forms disulfide bonds under physio-logical conditions is dynamic and reversible [
29]. The homolytic dissociation energies of disulfides generally are low (50–65 kcal mol
−1). The various nature of the groups flanking the disulfide bonds make the bonds respond to different stimuli (such as light, heat, mechanical force, and changes in the pH or redox state) [
30]. Consequently, disulfides can tune the properties of materials under mild reaction or stimulus conditions. The ease with which thiols can be converted to disulfides, and vice versa, renders disulfide bonds containing polymers as dynamic self-healing. Hydrogels cross-linked by disulfide bonds exhibit self-healing properties in addition to rapid gel formation [
31].
Xin Zhang et al. [
32] constructed injectable and rapid self-healing protein hydrogel by recombining the disulfide bond in Bovine serum albumin (BSA). By reducing the disulfide bond in BSA protein, the structure of BSA protein was unfolded. Without changing the secondary structure of BSA, the disulfide bond between each protein molecule was recombined to form a nontoxic, injectable, and rapid self-healing hydrogel. H
2O
2 can accelerate thiol oxidation at the damaged interface of BSA protein hydrogel so that the hydrogel can be wholly repaired within 1–2 min, and the repair efficiency reaches 100%. BSA protein hydrogel showed good fluid performance at 39% strain, which a pinhole injector could extrude. In another study, Hansen Yu et al. [
33] prepared injectable thermal responsive hydrogels that self-healing under weak acidic to alkaline conditions. The dynamic hydrogels prepared by mixing thiol functionalized F127 with disulfide-modified Polyethylene glycol(PEG) still have thermal responsiveness and undergo sol-gel transition under temperature change. In addition, hydrogels achieve self-healing under neutral or even mildly acidic conditions because the cyclic tension of cyclic disulfides increases the reactivity of disulfide bonds.
2.4. Diels-Alder(DA)
Otto Diels and his student, Kurt Alder, described the Diels–Alder (DA) reaction in 1928 [
34]. DA reaction is stereoselective, atom economical, and highly efficient; it is one of the most powerful methods to synthesize unsaturated six-membered rings [
35]. Six π electrons rearrange to form a six-membered ring product by DA reaction (
Figure 1g–i). DA reaction readily proceeds in water; it does not require expensive and potentially toxic solvents; and it can react at room temperature. Additionally, The DA reaction greatly accelerated in water by hydrogen bonding to the activated complex and enforced hydrophobic interactions between the reactants [
36,
37,
38,
39]. DA reaction includes normal electron-demand DA reaction, intramolecular DA reaction and hetero-DA reaction. Due to thermal responsiveness, covalent bond strength, high selectivity, high chemical yield, and lack of byproducts, DA reaction is widely used to prepare dynamic covalent hydrogels [
40]. In addition, DA reaction belongs to click chemistry, which is considered a kind of biocompatibility reaction and can realize substrate binding to specific biomolecules.
Zhao Wei et al. [
41] prepared dextran-l-poly (ethylene glycol) self-healing hydrogel by reversible DA reaction. The hydrogel was prepared using biocompatibility fulvene-modified hydrophilic dextran (Dex-FE) as the main polymer chain and dichloroacetic acid-modified polyethylene glycol (PEG-DiCMA) as the cross-linking agent to form the hydrogel network dextran-l-poly (ethylene glycol) self-healing hydrogel.
In addition to good biocompatibility, the dextran-l-poly (ethylene glycol) self-healing hydrogel was divided into two parts after 7 h to achieve self-healing at 37 °C. De-qiang Li et al. [
42] also prepared self-healing pectin/chitosan hydrogel by Diels–Alder reaction. Compared with pectin/chitosan composite hydrogel prepared by pure physical interaction (electrostatic interaction and molecular entanglement), the cross-linking density of self-healing pectin/chitosan hydrogel was greatly improved by DA reaction, and it had pH responsiveness and good self-healing performance (bearing 500 g weight without damage). At the same time, self-healing pectin/chitosan hydrogel can achieve higher drug loading efficiency, and the drug release mechanism can be controlled by adjusting the cross-linking density.
2.5. Hydrogen Bond
Hydrogen bonds play a crucial role in the water solubility of substances [
43], the stability of DNA double helix structure [
44], the secondary and tertiary structures of proteins, and the properties of solid polymers [
45]. Hydrogen bond (
Figure 2a) is formed by the attraction between the hydrogen atom on the polar X–H bond and the lone pair electrons of another atom with strong electronegativity and small atomic radius (such as F, N, O). The hydrogen bonds between molecules are easy to form and have different properties at different temperatures and pH values, and the change is reversible. When the outside world destroys the hydrogel, the molecular chain of the polymer has fluidity under the action of hydrogen bonds, thus giving it self-healing property. The binding strength of different hydrogen bonds is significantly different but is weak (0.25–15 kcal mol
−1) [
46]. However, when there are many hydrogen bonds in hydrogels, hydrogen bonds have a significant improvement on the mechanical properties of hydrogels, and the formation and dissociation of hydrogen bonds are very fast, which can realize the rapid healing of hydrogels.
Chen et al. [
47] synthesized tannic acid-lipoic acid (TATA) hydrogels by free radical nucleophilic addition reaction of the polyphenol-sulfur group and ring-opening polymerization of lipoic acid. Multiple hydrogen bonds formed between disulfide bonds, polyphenol residues, and carboxyl groups give hydrogel self-healing and injectability. Hydrogen bonding improves the self-healing time of TATA hydrogel and makes TATA hydrogel adhere to the wound quickly. X.X. Sun et al. [
48] made self-healing PVA-agar hydrogel with excellent mechanical properties by hydrogen bond interaction. After freeze–thaw cycles, hydrogen bonds formed between polyvinyl alcohol (PVA) molecules and between PVA molecules and agar molecules so that the hydrogel has self-healing properties. The healing hydrogel can withstand 2.3 kg weight and 5.0 MPa tensile strength, and the elongation at break can reach 450%.
2.6. Ion Interactions
The reversible electrostatic interactions between oppositely charged moieties ionic bonds can help hydrogel realize the self-healing (
Figure 2b) [
49,
50]. This interaction can occur between the transition metal ions with empty orbits and the groups containing lone pair electrons and between polymers with opposite charges and between the same charged polymers with opposite charges.
The ions interaction frequently makes the mechanical properties of hydrogels more prominent. Hamed Daemi et al. [
51] synthesized alginate-based supramolecular polyurethane (ASPU) with adjustable mechanical properties by the ion interaction between polyanionic alginate and polycationic polyurethane. The mechanical properties of ASPU can be adjusted by alginate content in an extensive range, and the tensile strength and Young’s modulus can reach 48 MPa and 93 MPa, respectively. In addition, ASPU has good self-healing ability and is divided into two parts of hydrogels, which can be reconnected after the 30 s of contact. W. Chen et al. [
52] combined sodium carboxymethyl cellulose (CMC) with PAA-Fe
3+ hydrogels to provide PAA-Fe
3+ hydrogels with high tensile strength (4.42 MPa), high toughness (dissipation energy up to 1.98 MJ/m
3), and good self-healing properties. The self-healing property of hydrogels is derived from the dynamic reconstruction of the metal ion coordination bond between polyacrylic acid (PAA) and CMC.
2.7. Host-Guest Interaction
As shown in
Figure 2c, subject–object interaction generally occurs between two or more chemicals. These chemicals cause molecular assembly by binding to noncovalent complimentary inclusions, allowing one part (guest) to be physically inserted and contained in another part (host). Because this interaction is maintained through noncovalent interactions (such as hydrogen bonds and ionic bonding, van der Waals and hydrophobic interactions), cross-linking is reversible and can provide self-healing properties for hydrogels.
Zhijun Ren et al. [
53] prepared double-net hydrogel by dynamic cross-linking of O-carboxymethyl chitosan (O-CMCS) and PVA main chain. The hydrogel has a variety of dynamic cross-linking (Schiff base, host–guest interaction, borate ester bond, and hydrogen bond). The hydrogel has rapid self-healing properties (self-healing without external stimulation for 10 s), tissue adhesion, water absorption, and mechanical properties. Beilin Zhang et al. [
54] prepared QCS-CD-AD/GO hydrogel through host-guest interaction with quaternary ammonium chitosan grafted cyclodextrin (QCS-CD), quaternary ammonium chitosan grafted adamantane (QCS-AD), and graphene oxide grafted cyclodextrin (GO-CD) polymer solutions. The hydrogel had an excellent antibacterial activity of QCS and photo-thermal and electrical properties of reduced graphene oxide (rGO). The host–guest interaction between CD and AD provides self-healing properties for supramolecular hydrogels. In addition, the hydrogel has stable mechanical properties, good biocompatibility, and NIR radiation-induced antibacterial activity.
2.8. Hydrophobic Interactions
Hydrophobic interactions are as important as hydrogen bonds in protein folding, the properties of solid polymers, and the interactions between molecules in different solvents. Hydrophobic interactions refers to the phenomenon that hydrophobic groups close to each other to avoid water. Hydrophobic interactions are slightly stronger than hydrogen bonds, but the Hydrophobic interaction can be fine-tuned by changing the shape of hydrophobic area and the number of hydrophobic groups. Hydrophobic interactions occur in the aggregation of a hydrophobic surface or hydrophobic medium. Waterborne polymer chains aggregate and associate in an aqueous solution to form dynamic cross-linking points [
55] (
Figure 2d). The compact hydrophobic structure is easy to assemble, and the hydrophobic association area is rapidly reformed when destroyed. The reversible decomposition of hydrophobic interactions can provide self-healing ability for hydrogels.
Yuting Wang et al. [
56] complexed sulfonated polyurethane (SPU) with PAA by Zn
2+ to prepare SPU-PAA/Zn self-healing hydrogel films with high mechanical strength and excellent elasticity. The tensile strength and toughness of the hydrogel films are 7.1 MPa and 30.4 MJm
−3, respectively. At the same time, the SPU-PAA/Zn hydrogel membrane can be wholly restored to the original shape after tensile stress release with a strain of less than 500%. The integrity and mechanical properties of the fractured hydrogel film can be self-repaired.
Siheng Li et al. [
57] prepared PAAm/PAA-Fe
3+/NaCl hydrogel by complexing polyacrylamide (PAAm) with Fe
3+ chelating polyacrylic acid (PAA-Fe
3+). The loading of NaCl in the hydrogel can generate hydrophobic regions to improve its mechanical strength and elasticity and endow the hydrogel with ionic conductivity up to 100% (0.72 S/m
−1). Due to the hydrogen bond and coordination interaction, as well as the synergistic effect of hydrophobic regions, the synthesized PAAm/PAA-Fe
3+/NaCl water.
3. Application of Self-Healing Hydrogels in Tissue Engineering
Self-healing hydrogel has good self-healing, fatigue resistance, and reusability and has hydrophilicity and responsiveness to environmental stimuli of conventional hydrogels. With researchers’ in-depth study of self-healing hydrogels, the application fields of self-healing hydrogels have significantly been expanded, showing excellent development potential in wound dressings and drug delivery, tissue engineering, bionic electronic skin wearable electronic equipment, and other fields. In this section, we summarize the recent application of self-healing hydrogels in tissue engineering, mainly including the self-healing mechanism of hydrogels, some mechanical properties, induction factors, and the characteristics of hydrogels. The recent research progress of hydrogels in bone, cartilage, skin, cardiovascular, sensor and other significant fields was summarized in the following contents.
3.1. Bone Repair
Through intervention, bone defects greater than the critical defect value caused by diseases such as bone tumors and fractures can be repaired [
58]. As a new biomaterial with controllable mechanical properties and biocompatibility, hydrogel is widely used in bone tissue engineering (BTE) as a scaffold for growth factor transport and cell adhesion (
Table 1). In bone tissue engineering, idealized hydrogel scaffold materials could provide three-dimensional living space for cells and regulate the morphology and function of tissue engineering cells. The hydrogel can effectively support protein absorption and cell adhesion so that cells grow following the prefabricated three-dimensional scaffold and are replaced by bone cells during the gradual degradation of the hydrogel to achieve bone repair [
59].
M. J et al. [
60] crosslinks
N-hydroxysuccinimide-poly(2-oxazoline)s (Pox-NHS) and amine-poly(2-oxazoline)s/alendronate-poly(2-oxazoline)s (Pox-Ale) through Ca
2+ interact to form a hydrogel network. In addition, Ca
2+ can promote the repair of bone tissue to some extent. The alendronate-functionalized polymer has strongly reinforced the adhesive properties of the hydrogels. Pox-Ale@POx-NHS hydrogel has great potential in bone tissue bonding. Additionally, W. Huang et al. [
61] increased catechol group on chitosan by chemical grafting. The catechol-conjugated chitosan (CS–C) and dialdehyde cellulose nanocrystal (DACNC)-formed hydrogel was by mild Schiff base reaction, catechol enabled the hydrogel to have adhesion, hemostasis, and defect repair functions.
Researchers also added influence factors to the three-dimensional structural system of hydrogels to promote bone tissue repair. L. Ma et al. [
62] doped hydroxyapatite in the oxidized alginate@carboxymethyl chitosan hydrogel as an induction factor for bone repair to improve bone repair performance (
Figure 3). Different from the physical doping of the former, Y. Li et al. [
63] used aldehyde Hyaluronic acid (AHA)@polyethyleneimine (PEI)@Acrylic acid (AA) as the carrier to add nano bioactive glass (BGN) by Schiff base cross-linking. BGN not only added the characteristics of bone differentiation and bone regeneration to hydrogels but also enhanced the physical properties of hydrogels by the new network of BGN in hydrogels.
X. Lu et al. [
64] prepared self-healing hydrogels by combining chitosan with silk fibroin (SF) through hydrogen bond, ion interaction (Mg
2+), amide bond. Chitosan@SF hydrogels delivered and released recombinant human bone morphogenic protein-2 (rhBMP-2)/rat bone marrow-derived MSCs (rBMSCs) using the three-site network structure of CS @SF hydrogels. D. Li et al. [
65] also achieved similar results on the matrix of Alginate dialdehyde@gelatin.
Table 1.
Self-healing hydrogel for bone repair.
Table 1.
Self-healing hydrogel for bone repair.
| Hydrogel Substrate | Self-Healing Mechanism | Self-Healing Cycle | Inductor | Binding Mode | Mechanical Property | Characteristics of Hydrogels | Ref. |
|---|
| AHA@PEI/AA | Schiff base/
Ion interaction | Completely healed after 12 h | Nanoscale bioactive glass | Schiff base | 2000 Pa
(at 88% Compression stress strain) | Antibacterial/enhanced osteogenic differentiation, skull regeneration/Drug delivery | [63] |
| CS/Silk fibroin(SF) | Hydrogen bond/
Ion interaction/
Amide bond | / | rhBMP-2/rBMSCs/Mg2+ | Immobilized with structure | 300 MPa
(Young’s modulus) | Immobilization cells delivery and implantation of stem cells/Drug delivery | [64] |
| Oxidized pullulan/poly(ethylene glycol)-Dex | Hydrazone | Two-part distribution uniform
within 24 h | Dexamethasone | Hydrazine | 2.53 kPa
(average storage modulus) | Antioxidant/anti-inflammatory/cell proliferative | [66] |
POx-NHS@amine-poly(2-oxazoline)s/
POx-Ale/Ca2+ | Hydrogen bond/
Ion interaction | recovery 107% within 10 min | CaP/soluble Ca2+ | Ion interaction | 200 kPa
(low storage moduli) | Bone cohesive | [60] |
| CS-C@DACNC | Schiff base | within 2 min | Catechol | Chemical grafting | 1402.1 Pa
(storage modulus) | Cohesive ability/promote bone repair | [61] |
| Alginate dialdehyde@gelatin | Ion interaction/
Schiff base | within 5 min | Demineralized bone matrix/BMSCs | Immobilized with structure | 112 kPa
(compressive strength) | Injectable/osteocalcin and VEGF highly expressed/Bone regeneration effect and bone defect repair | [65] |
| Oxidized alginate@carboxymethyl chitosan | Schiff base | Two-part distribution uniform within 1 h | Hydroxyapatite | Schiff base | 800 Pa
(storing elastic deformation energy) | Potential bone regeneration effect/Bone defect repair | [62] |
3.2. Cartilage Repair
Osteoarthritis and sports injury can cause articular cartilage lesions, but cartilage’s limited internal healing ability will lose cartilage [
67,
68]. The biomimetic microenvironment of self-healing hydrogel can promote cell migration, proliferation, and angiogenesis to repair cartilage tissue (
Table 2). Articular cartilage needs more frequent mechanical stress than bones, so hydrogel used in cartilage repair needs better mechanical new energy and self-healing performance. It can also match the dynamic loading microenvironment of natural cartilage, providing more possibilities for cartilage tissue repair.
To meet the specific needs of hydrogel in cartilage repair, S. Maiz-Fernández et al. [
69] selected chitosan and hyaluronic acid (HA) to construct hydrogel network by electrostatic interaction so that it has the possibility of loading a variety of drugs (the study also loaded with diclofenac and rifampicin). P. Baei et al. [
70] introduced catechol into sulfated alginate (SAlg) to bind chitosan (CS), which not only endows hydrogel cells with high affinity for adhesion, tissue integration, and bio-factor immobilization. Moreover, the hydrophobicity of cats reduced the swelling ratio of hydrogels and improved the mechanical properties.
In addition to the selection and modification of the hydrogel network matrix, some researchers added new systems to the hydrogel network. T. Zhou et al. [
71] used poly(ethylene glycol)-b-polythioketal-b-poly(ethylene glycol) (PEG-PTK-PEG) micelles as the carrier of dexamethasone (DA), combined with hydrazine grafted hyaluronic acid (HA-ADH) and aldehyde modified dextran (Dex-ALH) to achieve slow linear release of DA, reduce oxidative stress, and inhibit the occurrence of osteoarthritis (
Figure 4). P. Wang et al. [
72] added microcrystalline cellulose (MCC) to functional polyglutamic acid (γ-PGA) and sodium alginate (SA). MCC has many hydrogen bonds, which prolong the degradation time (125%) and reduce the expansion rate (470%).
Table 2.
Self-healing hydrogel for cartilage repair.
Table 2.
Self-healing hydrogel for cartilage repair.
| Hydrogel Substrate | Self-Healing Mechanism | Self-Healing Cycle | Inductor | Binding Mode | Mechanical Property | Characteristics of Hydrogels | Ref. |
|---|
| HA@ Hydrazide | Hydrazone | 2 h | Infliximab | Imine bonds | Tunable | Anti-inflammatory/
Drug delivery | [73] |
| HA-ADH@Dex-ALH | Schiff base | 10 min | Dexamethasone | Loaded on micelles | 0.39 kPa
(storage modulus) | Alleviated osteoarthritis/
Preventing cartilage extracellular matrix degeneration | [71] |
| γ-PGA@SA | Schiff base/
Hydrogen bond | Short time | Microcrystalline cellulose | Hydrogen bond | 60–144 kPa
(mechanical strength) | Promote cartilage matrix deposition/
Enhancement of hydrogel system | [72] |
| CS@HA | Ion interaction | 15 min | diclofenac and rifampicin | Electrostatic interaction | 0.023 MPa
(stress modulus) | Controlled release of drugs | [69] |
| SAlg@CS | Ion interaction | Fast (healing efficient 80%) | MSC and chondrocyte | Co-culture encapsulate | 421.45 kPa
(compressive strength) | Higher mechanical stability/
Cell adhesion/
Tissue integration | [70] |
3.3. Skin Repair
Skin is the largest organ of the human body, which can regulate body temperature and feel external stimuli and prevent pathogens from invading the body [
74]. However, direct contact with the outside world makes the skin vulnerable to damage. Self-repair function of the skin is often unable to repair itself when it exceeds the critical value [
75]. Skin self-healing is often accompanied by scars and loss of appendages such as hair and sweat glands [
76]. The inherent properties of hydrogel make hydrogel have potential application in wound dressings (
Table 3). The hydrogel with self-healing property can achieve self-healing after material damage and maintain the protective effect of the material on the skin damage area.
Recently, Ye Wu et al. [
77] designed caffeic acid-grafted ε-polylysine (CE) and phenylboronic acid-grafted oxidized dextran (POD) self-healing hydrogel with inherent antibacterial and antioxidant properties (
Figure 5). Delytic sodium (DS) particles were directly embedded in the main chain of hydrogels, while MF was encapsulated in the core of micelles. Different binding modes make the release curves of mangiferin (MF) (sustained-release within 7 d) and DS (release 58.6% within 24 h) highly consistent with the healing process of the wound after infection. Zhixin Ling et al. [
78] physically doped polydopamine nanoparticle (pDA-NPs) and enhanced the mechanical properties and expanded the pore size of CA hydrogel in the dynamic covalent cross-linked CA hydrogel based on Schiff base. The release of pDA-NPs effectively promoted the repair of large skin wounds, enhanced angiogenesis, and reduced scars.
In tissue engineering, conductive biomaterials can promote intercellular signal transduction and current transmission from external electrical stimulation, promoting cell migration and angiogenesis. HuanLei et al. [
79] prepared this hydrogel by adding tannic acid (TA) and human-like collagen (HLC) into the dynamic cross-linking network of polyvinyl alcohol (PVA) and borax hydrogel, in which borax played the role of cross-linking agent and ionic conductor. The addition of HLC and TA changed the cross-linking density and pH value of hydrogels, thus adjusting the adaptability of the PVA-borax matrix and endowing it with hemostasis, antibacterial, anti-inflammatory, cell proliferation, and collagen deposition. Similarly, Zhulong Tu et al. [
80] realized the conductive function of the material by adding graphene into the hydrogel system.
Table 3.
Self-healing hydrogel for skin repair.
Table 3.
Self-healing hydrogel for skin repair.
| Hydrogel Substrate | Self-Healing Mechanism | Self-Healing Cycle | Inductor | Binding Mode | Mechanical Property | Characteristics of Hydrogels | Ref. |
|---|
| L-arginine-A@-CHO-PEG-CHO | Schiff-base | 5 min | pDA-NPs | Physically doping | 1.1 kPa (storage modulus) | Swelling capacity | [78] |
| POD-@DS&Micelles | Schiff base/
borate ester bond | 2 min | MF/DS | Encapsulated into micelles | 2 kPa (storage modulus) | pH/ROS dual-responsiveness/
Spatiotemporal delivery | [77] |
| PVA@borax hydrogel | Hydrogen bond/
Borate ester bond | Within 30 min | Tannic acid/HLC | Borate bonds | 10 kPa (storage modulus) | Self-adaptive/Self-healing properties/Bioadhesion | [79] |
| Polypeptide@polydopamine | Schiff-base/
Hydrogen bond | 60 min | Graphene oxide | Hydrophobic interaction/
Hydrogen bond | 8 kPa (storage modulus) | Thermosensitive/Antibacterial/
Antioxidant/conductive | [80] |
| CMCS-CQDAG@ODex | Schiff base | 3 h | carbon quantum dots (CQDAG) | Schiff base | 11 kPa (storage modulus) | Antibiofilm/Low-drug resistance/Flexibility | [81] |
| PVA@silk fibroin@borax | Borate ester bond | 30 s | Tannic acid | Hydrogen bond | 4500 Pa (storage modulus) | Antibacterial/Flexibility/Plasticity/Bioadhesion/Easy stripping | [24] |
| COL-GG-PNIPAM-GO-borax | Diol-Borate ester bond | Within 3 min | / | / | 5 kPa (storage modulus) | Conductive/Thermo and NIR sensitive/Accelerated healing | [82] |
| HA-PBA@TA/AgNP hydrogel | Borate ester bond | 10 min | AgNP | Encapsulated into micelles | 425 Pa (storage modulus) | Dual stimuli responsive/antibacterial/Anti-oxidative | [83] |
3.4. Cardiac Repair
Due to insufficient blood supply, many cardiomyocytes will be lost, and relatively disordered fibrous tissues will occupy highly ordered cardiomyocytes. Excessive and sustained fibrosis deposition in the matrix disrupts electrical integrity and electrical signal conduction between health and infarction sites, leading to systolic and diastolic dysfunction and arrhythmia [
84]. In these cases, reconstruction of expected electrical pulse propagation in fibrotic tissues has become the most critical issue in myocardial infarction repair [
85]. Hydrogel-engineered heart patch can prevent heart failure after myocardial infarction and provide drugs or cells to promote damaged myocardial repair [
86]. Cardiac pulsation can easily lead to hydrogel damage and premature loss (
Table 4). Compared with traditional hydrogels, the drug delivery system’s self-healing hydrogels’ self-healing ability can ensure longer drug delivery and release time.
Rui Chen prepared a novel self-healing elastin to mimic peptide hydrogel (EMH) [
87]. Polydopamine (PDA) nanoparticles loaded with salvianolic acid B (SaB) can deliver SaB locally and enhance the self-healing ability of hydrogel. Prehydrogel (SaB-PDA/pre-EMH) has excellent biocompatibility and low viscosity, making it suitable for myocardial injection. Once injected into the myocardial infarction (MI) region, SaB-PDA/pre-EMH can form SaB-PDA/pre-EMH with tremendous mechanical strength under the action of upregulated glutamine transferase (TGase) in cardiac tissue after myocardial infarction.
Conductive scaffold materials have a positive effect on the behavior of myocardial cells. Song, XP et al. [
88] built PAA nanochannels inside porous ionic conductive hydrogel POG1 hydrogel, giving the hydrogel micro-heterogeneous electrical conductivity to make the cardiomyocytes (CM) inoculated in macroporous ionic conductive hydrogel (POG1) hydrogel show more oriented sarcomere (
Figure 6). In addition, POG1 hydrogel has suitable scalability (>500% strain) and compression (>85% strain), and its modulus is similar to that of the mammalian heart (30–500 kPa, Young’s modulus).
3.5. Nerve Injury Repair
Nerve damage can have devastating consequences for patients, including lifelong disability and death [
92,
93,
94]. The regenerative medicine treatment strategy is to achieve nerve repair by supplementing new nerve cells and inhibiting inflammatory response [
95]. However, direct transplanted neural stem cells do not have physical support in the injured area to fill the lesion and the lack of microenvironment for cell growth and differentiation, making the survival rate of neural stem cells after transplantation extremely low. Hydrogels have adjustable physical and chemical properties to fill irregular pathological cavities in the brain and provide a favorable microenvironment for the growth and proliferation of nerve cells [
96]. Self-healing hydrogels can achieve self-healing of nerves’ mechanical damage, maintain the integrated structure of nerve regeneration, and maintain long-term transplant structural integrity and persistence (
Table 5) (
Figure 7).
Lou J et al. [
97] developed injectable self-healing hybrid hydrogels using Fmoc grafted chitosan (FC) and Fmoc peptide (FI). At the same time, curcumin embedded and physically adsorbed can also be slowly released in the FC/FI system, making FC/FI-cur hydrogel accelerate neurite outgrowth of dorsal root ganglia (DRG) neurons. WenShi et al. [
98] prepared HA-PBA hydrogels by boronic ester dynamic covalent bond. The HA-PBA hydrogels can be used for reactive oxygen species responsive drug delivery and protected cells from ROS induced damage. In additionally, HA-PBA hydrogels could use for “direct-in-gel” printing to print 3D cell scaffolds.
Figure 7.
An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing [
99]. Reproduced with permission from Xu, J, Chem. Mater.; published by American Chemical Society, 2020.
Figure 7.
An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing [
99]. Reproduced with permission from Xu, J, Chem. Mater.; published by American Chemical Society, 2020.
In nerve repair, biomaterials with conductive activity show unique advantages in enhancing nerve activity and neuronal differentiation, which can significantly improve the possibility of nerve regeneration and myelin regeneration, to achieve complete overall recovery [
100,
101]. Biocompatible ECH dressing consisting of TA and polypyrrole(PPy) can attach to the high conductive film on the injured nerve and automatically enwrap it in the size-matched tubular structure. It forms a stable and intimate bridge coupling with the electrogenic nerve tissue to promote axonal regeneration and myelin regeneration [
102].
Table 5.
Self-healing hydrogel for nerve injury repair.
Table 5.
Self-healing hydrogel for nerve injury repair.
| Hydrogel Substrate | Self-Healing Mechanism | Self-Healing Cycle | Inductor | Binding Mode | Mechanical Property | Characteristics of Hydrogels | Ref. |
|---|
| Oxidized konjac glucomannan@Amino-PEI | Schiff base | 2 h | CNTs | Physical doping | more than 1 kPa
(storage modulus) | pH sensitivity/
Bio-printability/
Conductivity | [103] |
| FC@FI | Hydrogen bond | / | Curcumin | Embedding and
physical adsorption | 1 kPa
(storage modulus) | Injectable and
self-healing properties. | [97] |
| N-carboxyethyl chitosan@aldehyde- difunctional polyurethane | Schiff base | 30 min | chitosan-modified polypyrrole nanoparticle | Ion interaction | 250 Pa
(storage modulus) | Fast self-healing/
Tructural stability/
Durable elasticity. | [99] |
| L-glutamine amide derivative and benzaldehyde | Schiff base | 40 s | L-DOPA | Dissolve | 85 Pa
(storage modulus) | Rheological property/
Self-healing. | [104] |
| difunctional-PEG @glycol CS | Schiff base | 9 h | Cellulose nanofiber | UV crosslinking | 2 kPa
(storage modulus) | Biodegradable/
Tunable self-healing
properties. | [105] |
| TA | Ion interaction | / | Pyrroles/
ferric chloride hexahydrate | Coordination bonds | 846 ± 12 Pa
(storage modulus) | Porous/
Conductive/
Bioadhesion | [102] |
| HA-PBA@PVA | Borate ester bond | 10min | Neural progenitor cells | Encapsulated | 1155 Pa
(storage modulus) | pH sensitivity/
ROS responsive/
Anti-oxidative | [98] |
4. Conclusions and Recommendations
Based on the self-healing mechanism of self-healing hydrogels, the design strategies of self-healing hydrogels based on dynamic covalent and noncovalent interactions are summarized and sorted. The tissue engineering applications of self-healing hydrogels in bone, cartilage, skin, cardiovascular system, and nerve were also reviewed.
The formation mechanism of self-healing hydrogels determines the basic properties of hydrogels. Such as pH sensitivity of borate ester bond hydrogels, rapid healing of hydrogen bond hydrogels and strong mechanical properties of ionic bond hydrogels, but the physiological environment is a complex system. The single self-healing hydrogel based on dynamic covalent bond or dynamic noncovalent bond interaction mechanism has the problems of weak mechanical properties, harsh self-healing conditions and incomplete recovery. In previous comments, some researchers mentioned that through various dynamic covalent or noncovalent bonds, some researchers combined solid and rigid networks with weaker networks, usually made through reversible crosslinking, to improve mechanical properties. This is a hydrogel design idea with great potential.
When designing hydrogels for application in different tissues, researchers often choose to add different factors to the hydrogel network to improve the repair effect of tissues. For example, hydroxyapatite was added in bone repair hydrogel, neural cells were added in nerve repair hydrogel, and growth factors were added in skin repair hydrogel. Specific factors can significantly improve the functionality and repair effect of hydrogels. In complex in vivo mechanical environment, the release of physical doping or encapsulation factors is not controllable, which may bring some hidden dangers. If these hydrogel factors are combined to the three-dimensional network of hydrogel through chemical bonds (not only mentioned above), perhaps these factors will provide further improvement for the performance of hydrogel.
We do not recommend that researchers simply mix or encapsulate simple physical factors in the inherent three-dimensional structural network of hydrogels in the design process. In tissue engineering applications, the physical properties of hydrogel are not the only indicator. The mechanical properties required in different tissues (such as the difference between bone and skin) are different, but the hydrogels needed to achieve various functions in the same tissues (such as bone adhesion and filling of the bone defect) are different. In addition to the natural three-dimensional structure, hydrogel can achieve a variety of tissue repair factors (stem cells, drugs, bioactive substances, etc.) with local and precise release. Researchers can also modify the components of hydrogels in the matrix design of hydrogels to make hydrogels have more intelligent responses to the external environment (pH response, light response, thermal response, etc.). Hydrogels with broad diversity and adjustability exhibit more potential than other biomaterials in tissue engineering applications. In designing hydrogels, we should prepare different functional hydrogels for different application scenarios to further open the application of hydrogels.
Author Contributions
Conceptualization, L.Q.; methodology, L.Q.; software, L.Q.; validation, L.Q.; formal analysis, Y.X.; investigation, Y.X.; resources, L.Q.; data curation, X.W.; writing—original draft preparation, L.Q.; writing—review and editing, Y.X.; visualization, X.W.; supervision, Q.A.; project administration, Q.A.; funding acquisition, Q.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by grants from Sichuan Science and Technology Program (2020YFH0008) and National Key R&D Program of China (2020YFF0426289).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Niu, S.; Wang, L.; Lopez, J.; Chen, S.; Cai, Y.; Du, R.; Liu, Y.; Lai, J.-C.; Liu, L.; et al. An Elastic Autonomous Self-Healing Capacitive Sensor Based on a Dynamic Dual Crosslinked Chemical System. Adv. Mater. 2018, 30, 1801435. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-Healing of Chemical Gels Cross-Linked by Diarylbibenzofuranone-Based Trigger-Free Dynamic Covalent Bonds at Room Temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Brooks, W.; Abboud, K.; Sumerlin, B. Boronic Acid-Based Hydrogels Undergo Self-Healing at Neutral and Acidic pH. ACS Macro Lett. 2015, 4, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Pratama, P.A.; Sharifi, M.; Peterson, A.M.; Palmese, G.R. Room temperature self-healing thermoset based on the Diels-Alder reaction. ACS Appl. Mater. Interfaces 2013, 5, 12425–12431. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, T.; Zhu, Z.; Guo, L.; Li, X. Self-Healing Polycaprolactone Networks through Thermo-Induced Reversible Disulfide Bond Formation. Macromol. Rapid Commun. 2018, 39, e1800121. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Calabrese, E.; Barra, G.; Raimondo, M.; Sorrentino, A.; Binder, W.H.; Michael, P.; Rana, S. Self-healing epoxy nanocomposites via reversible hydrogen bonding. Compos. Part B Eng. 2019, 157, 1–13. [Google Scholar] [CrossRef]
- Andersen, A.; Krogsgaard, M.; Birkedal, H. Mussel-Inspired Self-Healing Double-Cross-Linked Hydrogels by Controlled Combination of Metal Coordination and Covalent Cross-Linking. Biomacromolecules 2018, 19, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-H.; Song, F.; Xue, J.; Qian, D.; Wang, X.-L.; Wang, Y.-Z. Mechanically strong and tough hydrogels with excellent anti-fatigue, self-healing and reprocessing performance enabled by dynamic metal-coordination chemistry. Polymer 2018, 153, 637–642. [Google Scholar] [CrossRef]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-Healing, Expansion-Contraction, and Shape-Memory Properties of a Preorganized Supramolecular Hydrogel through Host-Guest Interactions. Angew. Chem. Int. Ed. Engl. 2015, 54, 8984–8987. [Google Scholar] [CrossRef]
- Takashima, Y.; Yonekura, K.; Koyanagi, K.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Multifunctional Stimuli-Responsive Supramolecular Materials with Stretching, Coloring, and Self-Healing Properties Functionalized via Host–Guest Interactions. Macromolecules 2017, 50, 11. [Google Scholar] [CrossRef]
- Xia, N.N.; Xiong, X.M.; Rong, M.Z.; Zhang, M.Q.; Kong, F. Self-Healing of Polymer in Acidic Water toward Strength Restoration through the Synergistic Effect of Hydrophilic and Hydrophobic Interactions. ACS Appl. Mater. Interfaces 2017, 9, 37300–37309. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Dirksen, S.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of hydrazone formation and transimination: Implications for dynamic covalent chemistry. J. Am. Chem. Soc. 2006, 128, 15602–15603. [Google Scholar] [CrossRef] [PubMed]
- Huheey, J.E. Inorganic Chemistry; Principles of Structure and Reactivity; HarperCollins College Publishers: New York, NY, USA, 1972; pp. 139–152. [Google Scholar]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent Cross-Linked Polymer Gels with Reversible Sol−Gel Transition and Self-Healing Properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel Biocompatible Polysaccharide-Based Self-Healing Hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Dirksen, A.; Dawson, P.E. Rapid oxime and hydrazone ligations with aromatic aldehydes for biomolecular labeling. Bioconjug. Chem. 2008, 19, 2543–2548. [Google Scholar] [CrossRef] [Green Version]
- Apostolides, D.E.; Patrickios, C.S.; Leontidis, E.; Kushnir, M.; Wesdemiotis, C. Synthesis and characterization of reversible and self-healable networks based on acylhydrazone groups. Polym. Int. 2014, 63, 1558–1565. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Tang, W.; Du, S.; Yu, M.; Lu, H.; Tan, H.; Xing, X. A facile injectable carbon dot/oxidative polysaccharide hydrogel with potent self-healing and high antibacterial activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef]
- Bapat, A.P.; Sumerlin, B.S.; Sutti, A. Bulk network polymers with dynamic B-O bonds: Healable and reprocessable materials. Mater. Horiz. 2020, 7, 694–714. [Google Scholar] [CrossRef]
- Bull, S.D.; Davidson, M.G.; van den Elsen, J.M.H.; Fossey, J.S.; Jenkins, A.T.A.; Jiang, Y.-B.; Kubo, Y.; Marken, F.; Sakurai, K.; Zhao, J.; et al. Exploiting the Reversible Covalent Bonding of Boronic Acids: Recognition, Sensing, and Assembly. Acc. Chem. Res. 2013, 46, 312–326. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Liu, G.-J.; Ning, Z.-W.; Xing, G.-W. Boronic acid-based chemical sensors for saccharides. Carbohydr. Res. 2017, 452, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, H.; Theato, P. Glucose-Responsive Polymeric Micelles via Boronic Acid–Diol Complexation for Insulin Delivery at Neutral pH. Biomacromolecules 2019, 20, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lin, N.; He, Y.; Zuo, B. Self-Healing, Self-Adhesive Silk Fibroin Conductive Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 40013–40031. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Pan, R.; Liu, G.; Zeng, Y.; He, X.; Ma, Z.; Wei, Y.; Chen, S.; Yang, L.; Tao, L. A multi-responsive self-healing hydrogel for controlled release of curcumin. Polym. Chem. 2021, 12, 2457–2463. [Google Scholar] [CrossRef]
- Østergaard, H.; Tachibana, C.; Winther, J.R. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 2004, 166, 337–345. [Google Scholar] [CrossRef]
- Gething, M.J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Wu, Z.M.; Zhang, X.G.; Zheng, C.; Li, C.X.; Zhang, S.M.; Dong, R.N.; Yu, D.M. Disulfide-crosslinked chitosan hydrogel for cell viability and controlled protein release. Eur. J. Pharm. Sci. 2009, 37, 198–206. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef]
- Patenaude, M.; Smeets, N.M.B.; Hoare, T. Designing Injectable, Covalently Cross-Linked Hydrogels for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and fast self-healing protein hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.; Yang, H.; Peng, K.; Zhang, X. Injectable self-healing hydrogels formed via thiol/disulfide exchange of thiol functionalized F127 and dithiolane modified PEG. J. Mater. Chem. B 2017, 5, 4121–4127. [Google Scholar] [CrossRef] [PubMed]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Breslow, R.; Maitra, U.; Rideout, D. Selective diels-alder reactions in aqueous solutions and suspensions. Tetrahedron Lett. 1983, 24, 1901–1904. [Google Scholar] [CrossRef]
- Blokzijl, W.; Blandamer, M.J.; Engberts, J.B.F.N. Diels-Alder reactions in aqueous solutions. Enforced hydrophobic interactions between diene and dienophile. J. Am. Chem. Soc. 1991, 113, 4241–4246. [Google Scholar] [CrossRef]
- Meijer, A.; Otto, S.; Engberts, J.B.F.N. Effects of the Hydrophobicity of the Reactants on Diels−Alder Reactions in Water. J. Org. Chem. 1998, 63, 8989–8994. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert. Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Du, X.J.; Xu, F.; Zrinyi, M.; Osada, Y.; Li, F.; Chen, Y.M. Dextran-Based Self-Healing Hydrogels Formed by Reversible Diels–Alder Reaction under Physiological Conditions. Macromol. Rapid Commun. 2013, 34, 1464–1470. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, S.-Y.; Meng, Y.-J.; Guo, Z.-W.; Cheng, M.-M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef] [PubMed]
- Devereux, M.; Popelier, P. The Effects of Hydrogen-Bonding Environment on the Polarization and Electronic Properties of Water Molecules. J. Phys. Chem. A 2007, 111, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Sponer, J.; Leszczynski, J.; Hobza, P. Hydrogen bonding and stacking of DNA bases: A review of quantum-chemical ab initio studies. J. Biomol. Struct. Dyn. 1996, 14, 117–135. [Google Scholar] [CrossRef]
- Schroeter, J.; Felix, F. Melting cellulose. Cellulose 2005, 12, 159–165. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Nan, Y.; Okuro, K.; Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Yang, X.; Li, S.; Zhang, C.; Ma, Y.; Ma, Y.; Gao, P.; Gao, S.; Huang, X.-J. Tannic Acid-Thioctic Acid Hydrogel: A Novel Injectable Supramolecular Adhesive Gel for Wound Healing. Green Chem. 2021, 23, 1794–1804. [Google Scholar] [CrossRef]
- Sun, X.; Luo, C.; Luo, F. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.Q.; Chang, Q.; Jiang, J.Z.; Cai, J.; Wang, Q.; Luo, G.X.; Xing, M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Sun, S.T.; Mao, L.B.; Lei, Z.Y.; Yu, S.H.; Colfen, H. Hydrogels from Amorphous Calcium Carbonate and Polyacrylic Acid: Bio-Inspired Materials for “Mineral Plastics”. Angew. Chem.-Int. Ed. 2016, 55, 11765–11769. [Google Scholar] [CrossRef] [Green Version]
- Daemi, H.; Rajabi-Zeleti, S.; Sardon, H.; Barikani, M.; Khademhosseini, A.; Baharvand, H. A robust super-tough biodegradable elastomer engineered by supramolecular ionic interactions. Biomaterials 2016, 84, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Bu, Y.; Li, D.; Liu, C.; Chen, G.; Wan, X.; Li, N. High-strength, tough, and self-healing hydrogel based on carboxymethyl cellulose. Cellulose 2020, 27, 853–865. [Google Scholar] [CrossRef]
- Ren, Z.; Ke, T.; Ling, Q.; Zhao, L.; Gu, H. Rapid self-healing and self-adhesive chitosan-based hydrogels by host-guest interaction and dynamic covalent bond as flexible sensor. Carbohydr. Polym. 2021, 273, 118533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Head-Gordon, T. Is water structure around hydrophobic groups clathrate-like? Proc. Natl. Acad. Sci. USA 1995, 92, 8308–8312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Fang, X.; Li, S.; Pan, H.; Sun, J. Complexation of Sulfonate-Containing Polyurethane and Polyacrylic Acid Enables Fabrication of Self-Healing Hydrogel Membranes with High Mechanical Strength and Excellent Elasticity. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, H.; Wang, Y.; Sun, J. Polyelectrolyte complex-based self-healing, fatigue-resistant and anti-freezing hydrogels as highly sensitive ionic skins. J. Mater. Chem. A 2020, 8, 3667–3675. [Google Scholar] [CrossRef]
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Sánchez-Fernández, M.J.; Rutjes, J.; Félix Lanao, R.P.; Bender, J.C.M.E.; van Hest, J.C.M.; Leeuwenburgh, S.C.G. Bone-Adhesive Hydrogels Based on Dual Crosslinked Poly(2-oxazoline)s. Macromol. Biosci. 2021, 21, 2100257. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L.; Zhang, L. Noncompressible Hemostasis and Bone Regeneration Induced by an Absorbable Bioadhesive Self-Healing Hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Ma, L.; Su, W.; Ran, Y.; Ma, X.; Yi, Z.; Chen, G.; Chen, X.; Deng, Z.; Tong, Q.; Wang, X.; et al. Synthesis and characterization of injectable self-healing hydrogels based on oxidized alginate-hybrid-hydroxyapatite nanoparticles and carboxymethyl chitosan. Int. J. Biol. Macromol. 2020, 165 Pt A, 1164–1174. [Google Scholar] [CrossRef]
- Li, Y.; Ge, J.; Luo, M.; Niu, W.; Ling, X.; Xu, K.; Lin, C.; Lei, B.; Zhang, X. Elastomeric self-healing antibacterial bioactive nanocomposites scaffolds for treating skull defect. Appl. Mater. Today 2022, 26, 101254. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Li, J.; Sun, T.; Xiong, M. Recombinant Human Bone Morphogenic Protein-2 Immobilized Fabrication of Magnesium Functionalized Injectable Hydrogels for Controlled-Delivery and Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells in Femoral Head Necrosis Repair. Front. Cell Dev. Biol. 2021, 9, 723789. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Z.; Zhao, X.; Luo, Y.; Ou, Y.; Kang, P.; Tian, M. A bone regeneration strategy via dual delivery of demineralized bone matrix powder and hypoxia-pretreated bone marrow stromal cells using an injectable self-healing hydrogel. J. Mater. Chem. B 2021, 9, 479–493. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- McAdams, T.R.; Mithoefer, K.; Scopp, J.M.; Mandelbaum, B.R. Articular Cartilage Injury in Athletes. Cartilage 2010, 1, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D printable self-healing hyaluronic acid/chitosan polycomplex hydrogels with drug release capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef]
- Baei, P.; Daemi, H.; Mostafaei, F.; Azam Sayahpour, F.; Baharvand, H.; Baghaban Eslaminejad, M. A tough polysaccharide-based cell-laden double-network hydrogel promotes articular cartilage tissue regeneration in rabbits. Chem. Eng. J. 2021, 418, 129277. [Google Scholar] [CrossRef]
- Zhou, T.; Xiong, H.; Wang, S.Q.; Zhang, H.L.; Zheng, W.W.; Gou, Z.R.; Fan, C.Y.; Gao, C.Y. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis. Mater. Today Nano 2022, 17, 100164. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Y.; Ren, Y.; Yang, R.; Zhang, W.; Tan, X.; Xue, W.; Liu, S.; Li, S.; Chi, B. Dynamic regulable sodium alginate/poly(γ-glutamic acid) hybrid hydrogels promoted chondrogenic differentiation of stem cells. Carbohydr. Polym. 2022, 275, 118692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, C.; Liu, H.; Liu, H.; Feng, Y.; Li, Z.; Liu, H.; Wang, J.; Yang, B.; Lin, Q. Infliximab-based self-healing hydrogel composite scaffold enhances stem cell survival, engraftment, and function in rheumatoid arthritis treatment. Acta Biomater. 2021, 121, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Beanes, S.R.; Dang, C.; Soo, C.; Ting, K. Skin repair and scar formation: The central role of TGF-beta. Expert. Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, Z.; Deng, J.; Wang, Y.; Yuan, B.; Yang, X.; Lin, H.; Cao, J.; Zhu, X.; Zhang, X. A novel self-healing polydopamine-functionalized chitosan-arginine hydrogel with enhanced angiogenic and antibacterial activities for accelerating skin wound healing. Chem. Eng. J. 2021, 420, 130302. [Google Scholar] [CrossRef]
- Lei, H.; Fan, D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021, 421, 129578. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, M.; Wang, M.; Shao, Z.; Jiang, X.; Wang, K.; Yao, Z.; Yang, S.; Zhang, X.; Gao, W.; et al. Engineering Bioactive M2 Macrophage-Polarized Anti-Inflammatory, Antioxidant, and Antibacterial Scaffolds for Rapid Angiogenesis and Diabetic Wound Repair. Adv. Funct. Mater. 2021, 31, 2100924. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Yang, X.; Du, S.; Tang, W.; Cao, W.; Zhou, J.; Gong, X.; Xing, X. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021, 403, 126387. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, F.; Tang, L.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; Hu, X.; Lin, S.; Ding, C. Super-ductile, injectable, fast self-healing collagen-based hydrogels with multi-responsive and accelerated wound-repair properties. Chem. Eng. J. 2021, 405, 126756. [Google Scholar] [CrossRef]
- Shi, W.; Kong, Y.; Su, Y.; Kuss, M.A.; Jiang, X.; Li, X.; Xie, J.; Duan, B. Tannic acid-inspired, self-healing, and dual stimuli responsive dynamic hydrogel with potent antibacterial and anti-oxidative properties. J. Mater. Chem. B 2021, 9, 7182–7195. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Jackman, C.P.; Ganapathi, A.M.; Asfour, H.; Qian, Y.; Allen, B.W.; Li, Y.; Bursac, N. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 2018, 159, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Khattab, A.; Islam, M.A.; Hweij, K.A.; Zeitouny, J.; Waters, R.; Sayegh, M.; Hossain, M.M.; Paul, A. Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2, 1500122. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhu, C.; Xu, L.; Gu, Y.; Ren, S.; Bai, H.; Zhou, Q.; Liu, X.; Lu, S.; Bi, X.; et al. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials 2021, 274, 120855. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Theato, P.; Huang, C.F.; Hsu, S.H. A 3D-printable, glucose-sensitive and thermoresponsive hydrogel as sacrificial materials for constructs with vascular-like channels. Appl. Mater. Today 2020, 20, 100778. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.G.; Chen, M.; Xi, Y.W.; Cheng, W.; Mao, C.; Xu, T.Z.; Zhang, X.X.; Lin, C.; Gao, W.Y.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, D.; Ma, Z.J.; Liu, K.; Xue, K.; Li, H.Y. An effective strategy for preparing macroporous and self-healing bioactive hydrogels for cell delivery and wound healing. Chem. Eng. J. 2021, 425, 130677. [Google Scholar] [CrossRef]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Wolfe, D.L. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, Y.; Li, X.; Zhang, Q. The progress of biomaterials in peripheral nerve repair and regeneration. J. Neurorestoratology 2020, 8, 252–269. [Google Scholar] [CrossRef]
- Javed, R.; Ao, Q. Nanoparticles in peripheral nerve regeneration: A mini review. J. Neurorestoratology 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Jarrin, S.; Cabré, S.; Dowd, E. The potential of biomaterials for central nervous system cellular repair. Neurochem. Int. 2021, 144, 104971. [Google Scholar] [CrossRef]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.-L.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef]
- Xu, J.; Wong, C.-W.; Hsu, S.-H. An Injectable, Electroconductive Hydrogel/Scaffold for Neural Repair and Motion Sensing. Chem. Mater. 2020, 32, 10407–10422. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Qian, Y.; Cheng, Y.; Song, J.; Xu, Y.; Yuan, W.E.; Fan, C.; Zheng, X. Mechano-Informed Biomimetic Polymer Scaffolds by Incorporating Self-Powered Zinc Oxide Nanogenerators Enhance Motor Recovery and Neural Function. Small 2020, 16, e2000796. [Google Scholar] [CrossRef]
- Liu, C.; Fan, L.; Tian, Z.; Wen, H.; Zhou, L.; Guan, P.; Luo, Y.; Chan, C.; Tan, G.; Ning, C.; et al. Self-curling electroconductive nerve dressing for enhancing peripheral nerve regeneration in diabetic rats. Bioact. Mater. 2021, 6, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Han, L.; Zhang, X.L.; Cao, L.; Hu, K.; Li, L.H.; Wei, Y. 3D bioprinting of an electroactive and self-healing polysaccharide hydrogels. J. Tissue Eng. Regen. Med. 2022, 16, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Rodrigo, A.C.; Patterson, A.K.; Hawkins, K.; Aly, M.M.S.; Sun, J.; Al Jamal, K.T.; Smith, D.K. Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Adv. Sci. 2021, 8, e2101058. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 25. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).