Anion Exchange Membranes for Fuel Cells Based on Quaternized Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers with Spacer-Sidechain Design
Abstract
:1. Introduction
2. Methods
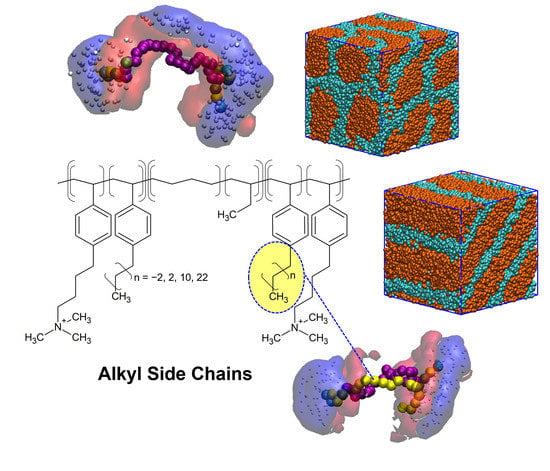
2.1. The Chemical Structure and Coarse-Grained Model for the Designed Polymers
2.2. The Force Field Parameters for Dissipative Particle Dynamics Simulations
2.3. The Coarse-Grained Model for Hydroxide Ions
3. Results and Discussions
3.1. Nanostructure and Conductivity of Anion Exchange Membranes
3.2. The Effects of the Alkyl Side Chains

3.3. The Effects of the Side-Chain Tethering Style
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EERE. Multiyear Research, Development and Demonstration Plan; EERE: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Marx, D.; Chandra, A.; Tuckerman, M.E. Aqueous Basic Solutions: Hydroxide Solvation, Structural Diffusion, and Comparison to the Hydrated Proton. Chem. Rev. 2010, 110, 2174–2216. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Maurya, S.; Shin, S.H.; Kim, Y.; Moon, S.H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Zhang, H.W.; Chen, D.Z.; Xianze, Y.; Yin, S.B. Anion-Exchange Membranes for Fuel Cells: Synthesis Strategies, Properties and Perspectives. Fuel Cells 2015, 15, 761–780. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.X.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef] [Green Version]
- Noh, S.; Jeon, J.Y.; Adhikari, S.; Kim, Y.S.; Bae, C. Molecular Engineering of Hydroxide Conducting Polymers for Anion Exchange Membranes in Electrochemical Energy Conversion Technology. Acc. Chem. Res. 2019, 52, 2745–2755. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 13. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 35. [Google Scholar] [CrossRef]
- Arges, C.G.; Zhang, L. Anion Exchange Membranes’ Evolution toward High Hydroxide Ion Conductivity and Alkaline Resiliency. ACS Appl. Energ. Mater. 2018, 1, 2991–3012. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.S.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Diesendruck, C.E.; Dekel, D.R. Water—A key parameter in the stability of anion exchange membrane fuel cells. Curr. Opin. Electrochem. 2018, 9, 173–178. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Lim, H.; Lee, B.; Yun, D.; Al Munsur, A.Z.; Chae, J.E.; Lee, S.Y.; Kim, H.J.; Nam, S.Y.; Park, C.H.; Kim, T.H. Poly(2,6-dirnethyl-1,4-phenylene oxide)s with Various Head Groups: Effect of Head Groups on the Properties of Anion Exchange Membranes. Acs Appl. Mater. Interfaces 2018, 10, 41279–41292. [Google Scholar] [CrossRef]
- Fujimoto, C.; Kim, D.-S.; Hibbs, M.; Wrobleski, D.; Kim, Y.S. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells. J. Membr. Sci. 2012, 423-424, 438–449. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Sturgeon, M.R.; Long, H.; Pivovar, B.S.; Bae, C. Thermochemical Stability Study of Alkyl-Tethered Quaternary Ammonium Cations for Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2017, 164, F1279–F1285. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef] [Green Version]
- Amel, A.; Zhu, L.; Hickner, M.; Ein-Eli, Y. Influence of Sulfone Linkage on the Stability of Aromatic Quaternary Ammonium Polymers for Alkaline Fuel Cells. J. Electrochem. Soc. 2014, 161, F615–F621. [Google Scholar] [CrossRef] [Green Version]
- Al Munsur, A.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.H. Quaternary ammonium-functionalized hexyl bis(quaternary ammonium)-mediated partially crosslinked SEBSs as highly conductive and stable anion exchange membranes. Int. J. Hydrog. Energy 2020, 45, 15658–15671. [Google Scholar] [CrossRef]
- Al Munsur, A.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.H. Hydrophobic-hydrophilic comb-type quaternary ammonium-functionalized SEBS copolymers for high performance anion exchange membranes. J. Membr. Sci. 2020, 599, 10. [Google Scholar] [CrossRef]
- Castaneda, S.; Ribadeneira, R. Theoretical Description of the Structural Characteristics of the Quaternized SEBS Anion-Exchange Membrane Using DFT. J. Phys. Chem. C 2015, 119, 28235–28246. [Google Scholar] [CrossRef]
- Castaneda, S.; Ribadeneira, R. Description of Hydroxide Ion Structural Diffusion in a Quaternized SEBS Anion Exchange Membrane Using Ab Initio Molecular Dynamics. J. Phys. Chem. C 2020, 124, 9834–9851. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.H.; Xu, R.W.; Zhang, S.; Wu, Y.X. Cross-Linked Quaternized Poly(styrene-b-(ethylene-co-butylene)-b-styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. Acs Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef]
- Gao, X.Q.; Yu, H.M.; Qin, B.W.; Jia, J.; Hao, J.K.; Xie, F.; Shao, Z.G. Enhanced water transport in AEMs based on poly(styrene-ethylene-butylene-styrene) triblock copolymer for high fuel cell performance. Polym. Chem. 2019, 10, 1894–1903. [Google Scholar] [CrossRef]
- Gupta, G.; Scott, K.; Mamlouk, M. Soluble Polystyrene-b-poly (ethylene/butylene)-b-polystyrene Based Ionomer for Anion Exchange Membrane Fuel Cells Operating at 70 degrees C. Fuel Cells 2018, 18, 137–147. [Google Scholar] [CrossRef]
- Hao, J.K.; Gao, X.Q.; Jiang, Y.Y.; Zhang, H.J.; Luo, J.S.; Shao, Z.G.; Yi, B.L. Crosslinked high-performance anion exchange membranes based on poly (styrene-b-(ethylene-co-butylene)-b-styrene). J. Membr. Sci. 2018, 551, 66–75. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.; Han, J.; Maurya, S.; Mohanty, A.D.; Tian, D.; Saikia, N.; Hickner, M.A.; Ryu, C.Y.; Tuckerman, M.E.; et al. Synthesis of Aromatic Anion Exchange Membranes by Friedel-Crafts Bromoalkylation and Cross-Linking of Polystyrene Block Copolymers. Macromolecules 2019, 52, 2139–2147. [Google Scholar] [CrossRef]
- Lee, M.T. Designing Highly Conductive Block Copolymer-Based Anion Exchange Membranes by Mesoscale Simulations. J. Phys. Chem. B 2021, 125, 2729–2740. [Google Scholar] [CrossRef]
- Luo, X.B.; Liu, H.J.; Bae, C.; Tuckerman, M.E.; Hickner, M.A.; Paddison, S.J. Mesoscale Simulations of Quaternary Ammonium-Tethered Triblock Copolymers: Effects of the Degree of Functionalization and Styrene Content. J. Phys. Chem. C 2020, 124, 16315–16323. [Google Scholar] [CrossRef]
- Luo, X.B.; Paddison, S.J. DPD simulations of anion exchange membrane: The effect of an alkyl spacer on the hydrated morphology. Solid State Ion. 2019, 339, 9. [Google Scholar] [CrossRef]
- Sepehr, F.; Liu, H.J.; Luo, X.B.; Bae, C.S.; Tuckerman, M.E.; Hicknee, M.A.; Paddison, S.J. Mesoscale Simulations of Anion Exchange Membranes Based on Quaternary Ammonium Tethered Triblock Copolymers. Macromolecules 2017, 50, 4397–4405. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Parrondo, J.; Ramani, V. Polystyrene-Block-Poly(ethylene-ran-butylene)-Block-Polystyrene Triblock Copolymer Separators for a Vanadium-Cerium Redox Flow Battery. J. Electrochem. Soc. 2017, 164, F372–F378. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Parrondo, J.; Ramani, V. Anion Exchange Membranes Based on Polystyrene-Block-Poly(ethylene-ran-butylene)-Block-Polystyrene Triblock Copolymers: Cation Stability and Fuel Cell Performance. J. Electrochem. Soc. 2017, 164, F1216–F1225. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Liu, Q.L.; Broadwell, I.; Zhu, A.M.; Xiong, Y.; Tu, X.P. Anion exchange membranes based on quaternized polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene for direct methanol alkaline fuel cells. J. Membr. Sci. 2010, 349, 237–243. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Luo, X.B.; Paddison, S.J. DPD simulations of anion exchange membranes functionalized with various cationic groups and associated anions. Solid State Ion. 2019, 340, 8. [Google Scholar] [CrossRef]
- Heck, B.; Arends, P.; Ganter, M.; Kressler, J.; Stühn, B. SAXS and TEM Studies on Poly(styrene)-block-poly(ethene-co-but-1-ene)-block-poly(styrene) in Bulk and at Various Interfaces. Macromolecules 1997, 30, 4559–4566. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.S.; Zhou, J.; Xu, Q.M.; Chu, D.; Chen, R.R. Novel nanostructured high-performance anion exchange ionomers for anion exchange membrane fuel cells. J. Power Sources 2012, 202, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Guo, J.S.; Chu, D.; Chen, R.R. Impacts of anion-exchange-membranes with various ionic exchange capacities on the performance of H-2/O-2 fuel cells. J. Power Sources 2012, 219, 272–279. [Google Scholar] [CrossRef]
- Gupta, G.; Scott, K.; Mamlouk, M. Performance of polyethylene based radiation grafted anion exchange membrane with polystyrene-b-poly (ethylene/butylene)-b-polystyrene based ionomer using NiCo2O4 catalyst for water electrolysis. J. Power Sources 2018, 375, 387–396. [Google Scholar] [CrossRef]
- Dekel, D.R.; Arnar, M.; Willdorf, S.; Kosa, M.; Dhara, S.; Diesendruck, C.E. Effect of Water on the Stability of Quaternary Ammonium Groups for Anion Exchange Membrane Fuel Cell Applications. Chem. Mater. 2017, 29, 4425–4431. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.X.; Wang, X.Q.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Quaternized triblock polymer anion exchange membranes with enhanced alkaline stability. J. Membr. Sci. 2017, 541, 358–366. [Google Scholar] [CrossRef]
- Barnett, A.; Lu, J.; Molinero, V. Width and Clustering of Ion-Conducting Channels in Fuel Cell Membranes Are Insensitive to the Length of Ion Tethers. J. Phys. Chem. C 2021, 125, 27693–27702. [Google Scholar] [CrossRef]
- Lu, J.B.; Barnett, A.; Molinero, V. Effect of Polymer Architecture on the Nanophase Segregation, Ionic Conductivity, and Electro-Osmotic Drag of Anion Exchange Membranes. J. Phys. Chem. C 2019, 123, 8717–8726. [Google Scholar] [CrossRef]
- Barnett, A.; Lu, J.; Molinero, V. Mechanism of Facilitation of Ion Mobility in Low-Water-Content Fuel Cell Membranes. J. Phys. Chem. C 2021, 125, 27703–27713. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide-Polyaniline-Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, X.; Paddison, S.J. Coarse-Grained Modeling of Ion-Containing Polymers. Chem. Rev. 2022, 122, 10710–10745. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef]
- Lee, M.T. Designing Anion Exchange Membranes with Enhanced Hydroxide Ion Conductivity by Mesoscale Simulations. J. Phys. Chem. C 2020, 124, 4470–4482. [Google Scholar] [CrossRef]
- Hibbs, M.R. Alkaline stability of poly(phenylene)-based anion exchange membranes with various cations. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1736–1742. [Google Scholar] [CrossRef]
- Sepehr, F.; Paddison, S.J. Dissipative Particle Dynamics interaction parameters from ab initio calculations. Chem. Phys. Lett. 2016, 645, 20–26. [Google Scholar] [CrossRef]
- Liu, H.J.; Cavaliere, S.; Jones, D.J.; Roziere, J.; Paddison, S.J. Scaling Behavior of Nafion with Different Model Parameterizations in Dissipative Particle Dynamics Simulations. Macromol. Theory Simul. 2018, 27, 9. [Google Scholar] [CrossRef]
- Liu, H.J.; Cavaliere, S.; Jones, D.J.; Roziere, J.; Paddison, S.J. Morphology of Hydrated Nafion through a Quantitative Cluster Analysis: A Case Study Based on Dissipative Particle Dynamics Simulations. J. Phys. Chem. C 2018, 122, 13130–13139. [Google Scholar] [CrossRef]
- Espanol, P.; Warren, P. Statistical-Mechanics of Dissipative Particle Dynamics. Europhys. Lett. 1995, 30, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.T.; Vishnyakov, A.; Neimark, A.V. Calculations of Critical Micelle Concentration by Dissipative Particle Dynamics Simulations: The Role of Chain Rigidity. J. Phys. Chem. B 2013, 117, 10304–10310. [Google Scholar] [CrossRef]
- Lee, M.T.; Mao, R.F.; Vishnyakov, A.; Neimark, A.V. Parametrization of Chain Molecules in Dissipative Particle Dynamics. J. Phys. Chem. B 2016, 120, 4980–4991. [Google Scholar] [CrossRef]
- Lee, M.T.; Vishnyakov, A.; Neimark, A.V. Coarse-grained model of water diffusion and proton conductivity in hydrated polyelectrolyte membrane. J. Chem. Phys. 2016, 144. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Lee, M.-T.; Neimark, A.V. Prediction of the Critical Micelle Concentration of Nonionic Surfactants by Dissipative Particle Dynamics Simulations. J. Phys. Chem. Lett. 2013, 4, 797–802. [Google Scholar] [CrossRef]
- Lee, M.T. Exploring Side-Chain Designs for Enhanced Ion Conductivity of Anion-Exchange Membranes by Mesoscale Simulations. J. Phys. Chem. C 2019, 123, 10802–10815. [Google Scholar] [CrossRef]
- Tuckerman, M.E.; Marx, D.; Parrinello, M. The nature and transport mechanism of hydrated hydroxide ions in aqueous solution. Nature 2002, 417, 925–929. [Google Scholar] [CrossRef]
- Tuckerman, M.E.; Chandra, A.; Marx, D. Structure and dynamics of OH-(aq). Acc. Chem. Res. 2006, 39, 151–158. [Google Scholar] [CrossRef]
- Ma, Z.H.; Tuckerman, M.E. On the connection between proton transport, structural diffusion, and reorientation of the hydrated hydroxide ion as a function of temperature. Chem. Phys. Lett. 2011, 511, 177–182. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’s Physical Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Lee, M.T.; Vishnyakov, A.; Neimark, A.V. Modeling Proton Dissociation and Transfer Using Dissipative Particle Dynamics Simulation. J. Chem. Theory Comput. 2015, 11, 4395–4403. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef] [Green Version]
- The Fuel Cell Technologies Program Multi-Year Research, Development, and Demonstration Plan (MYRD&D Plan). 2012, United State. Available online: https://www.energy.gov/sites/prod/files/2014/12/f19/fcto_myrdd_full_document.pdf (accessed on 26 June 2022).
- Kamel, M.S.A.; Mohamed, H.F.M.; Abdel-Hamed, M.O.; Abdel-Hady, E.E. Characterization and evaluation of Nafion HP JP as proton exchange membrane: Transport properties, nanostructure, morphology, and cell performance. J. Solid State Electrochem. 2019, 23, 2639–2656. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Kobayashi, Y.; Kuroda, C.S.; Takimoto, N.; Ohira, A. Free volume, oxygen permeability, and uniaxial compression storage modulus of hydrated biphenol-based sulfonated poly(arylene ether sulfone). J. Membr. Sci. 2010, 360, 84–89. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Ohira, A.; Kobayashi, Y. Free Volume and Oxygen Permeability in Polymers Related to Polymer Electrolyte Fuel Cells. Mater. Sci. Forum 2009, 607, 58–60. [Google Scholar] [CrossRef]
- Sarkisov, L.; Bueno-Perez, R.; Sutharson, M.; Fairen-jimenez, D. Material Informatics with PoreBlazer v4.0 and CSD MOF Database. ChemRxiv 2020. Preprint. [Google Scholar] [CrossRef]
- Easteal, A.J.; Price, W.E.; Woolf, L.A. Diaphragm cell for high-temperature diffusion measurements—Tracer diffusion-coefficients for water to 363-K. J. Chem. Soc. Faraday Trans. I 1989, 85, 1091–1097. [Google Scholar] [CrossRef]
- Gonzalez-Melchor, M.; Mayoral, E.; Velazquez, M.E.; Alejandre, J. Electrostatic interactions in dissipative particle dynamics using the Ewald sums. J. Chem. Phys. 2006, 125. [Google Scholar] [CrossRef]
- Anderson, R.L.; Bray, D.J.; Del Regno, A.; Seaton, M.A.; Ferrante, A.S.; Warren, P.B. Micelle Formation in Alkyl Sulfate Surfactants Using Dissipative Particle Dynamics. J. Chem. Theory Comput. 2018, 14, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [Green Version]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid lonomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Lyubartsev, A.P.; Laaksonen, A.M. DynaMix—A scalable portable parallel MD simulation package for arbitrary molecular mixtures. Comput. Phys. Commun. 2000, 128, 565–589. [Google Scholar] [CrossRef]









| Repulsion Parameters | |||||
|---|---|---|---|---|---|
| aij | B/S a | C | P | W | A |
| B/S | 25.0 | ||||
| C | 46.5 | 25.0 | |||
| P | 30.4 | 41.2 | 25.0 | ||
| W | 46.5 | 41.2 | 46.5 | 25.0 | |
| A | 25.0 | 25.0 | 25.0 | −8.3 | 25.0 |
| Bond Parameters | |||||
| Backbone b | K(12) | r0(12) | K(13) | r0(13) | |
| 240.0 | 0.6 | 12.0 | 1.7 | ||
| Side chain c | K(12) | r0(12) | K(13) | r0(13) | |
| 80.0 | 0.8 | 40.0 | 1.6 | ||
| Polymer | IEC [mmol/g] | λ a | WU b (wt%) | DW/DW_bulk c | DA/DW c | σ d [mS/cm] | dmin e (nm) | Err. | dmax e (nm) | Err. f |
|---|---|---|---|---|---|---|---|---|---|---|
| SEBS–C4Q–C0 | 1.72 | 10 | 31% | 0.48 | 1.96 | 24.3 | 2.0 | 0.1 | 3.0 | 0.1 |
| SEBS–C4Q–C0 | 1.72 | 20 | 62% | 0.65 | 2.31 | 32.7 | 2.8 | 0.1 | 4.1 | 0.1 |
| SEBS–C4Q–C0 | 1.72 | 30 | 93% | 0.69 | 2.88 | 36.8 | 3.6 | 0.1 | 5.0 | 0.2 |
| SEBS–C4Q–C4 | 1.64 | 10 | 30% | 0.52 | 1.85 | 24.0 | 2.1 | 0.1 | 3.0 | 0.1 |
| SEBS–C4Q–C4 | 1.64 | 20 | 59% | 0.58 | 2.74 | 33.0 | 2.9 | 0.0 | 4.1 | 0.1 |
| SEBS–C4Q–C4 | 1.64 | 30 | 89% | 0.69 | 2.92 | 36.2 | 3.5 | 0.2 | 5.1 | 0.4 |
| SEBS–C4Q–C12 | 1.51 | 10 | 27% | 0.44 | 1.94 | 19.9 | 2.1 | 0.1 | 2.9 | 0.0 |
| SEBS–C4Q–C12 | 1.51 | 20 | 54% | 0.58 | 2.44 | 28.0 | 2.8 | 0.2 | 4.0 | 0.1 |
| SEBS–C4Q–C12 | 1.51 | 30 | 81% | 0.65 | 2.79 | 31.1 | 3.6 | 0.1 | 5.1 | 0.2 |
| SEBS–C4Q–C24 | 1.34 | 10 | 24% | 0.47 | 1.82 | 18.6 | 2.1 | 0.1 | 3.0 | 0.1 |
| SEBS–C4Q–C24 | 1.34 | 20 | 48% | 0.51 | 2.50 | 23.4 | 2.3 | 0.3 | 3.3 | 0.6 |
| SEBS–C4Q–C24 | 1.34 | 30 | 72% | 0.63 | 2.69 | 27.3 | 2.5 | 0.6 | 3.7 | 1.1 |
| SEBS–C4Q–C12* | 1.46 | 10 | 26% | 0.49 | 1.74 | 20.5 | 2.2 | 0.1 | 3.1 | 0.1 |
| SEBS–C4Q–C12* | 1.46 | 20 | 53% | 0.60 | 2.53 | 30.6 | 2.9 | 0.0 | 4.1 | 0.1 |
| SEBS–C4Q–C12* | 1.46 | 30 | 79% | 0.67 | 2.77 | 32.5 | 3.4 | 0.1 | 5.2 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-G.; Lee, M.-T. Anion Exchange Membranes for Fuel Cells Based on Quaternized Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers with Spacer-Sidechain Design. Polymers 2022, 14, 2860. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142860
Chen Q-G, Lee M-T. Anion Exchange Membranes for Fuel Cells Based on Quaternized Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers with Spacer-Sidechain Design. Polymers. 2022; 14(14):2860. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142860
Chicago/Turabian StyleChen, Qun-Gao, and Ming-Tsung Lee. 2022. "Anion Exchange Membranes for Fuel Cells Based on Quaternized Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers with Spacer-Sidechain Design" Polymers 14, no. 14: 2860. https://0-doi-org.brum.beds.ac.uk/10.3390/polym14142860